Good Manufacturing Practices Regulations For Medical Devices
What are good manufacturing practice (GMP) regulations?
Good manufacturing practice (GMP) regulations were first proposed in the United States in 1963, following the 1962 Kefauver–Harris amendment on drug efficacy. GMP regulations cover requirements for the manufacturing, packaging, and distribution of products in contact with humans and animals. The need for GMP regulations arose because patients couldn’t detect a defective or low-quality product before it was too late. Thus, GMP regulations were put in place for patient protection. GMP is especially critical for ensuring that pharmaceutical products are safe and effective. Each GMP regulation and guidance statement aims to meet one or more of the five attributes listed below.
GMP compliance ensures that products taken by or administered to humans and animals meet or exceed minimum requirements for:
- Safety
- Identity
- Strength
- Purity
- Quality
It took over 15 years for the Food and Drug Association (FDA) and the U.S. pharmaceutical industry to agree on terminology and descriptions in the GMP regulations. GMP terminology and descriptions took so long to be agreed upon because the pharma industry fought vigorously for the GMP regulations not to be substantive and give the pharma industry freedom to interpret and apply the regulations as they saw fit. However, when GMPs became official in 1978, FDA inspections began, and the FDA started to enforce their compliance. As a result, the pharma industry found they needed to understand better how the FDA was interpreting and enforcing the regulations. FDA guidance documents began to be issued to fill this knowledge gap. These guidance documents gave the industry additional information on the FDA’s expectations, especially in areas of validation and documentation. Table 25-1 below contains a partial list of FDA Guidance documents. FDA guidance documents are not legally binding. However, the FDA requires sound justification if the industry chooses not to follow the guidance documents.
Examples of Relevant FDA Guidance Documents for GMP Compliance
- Guidanoe for the submission of documentation for sterilization prooess validation in applications for human and veterinary drug products
- Guidance for industry: sterile drug products produced by aseptic processing, current good manufacturing practice
- Guideline for validation of Limulus Amebocyte Lysate Test as an end product endotoxin test for human and animal parenteral drugs, biological products, and medical devices
- Compliance program guidance manual 7356.002 A, sterile drug process inspections
- Guide to inspections of lyophilization of parenterals
- Guide to inspections of high purity water systems
- Guide to inspections of microbiological pharmaceutical quality control laboratories
- Guide to inspections of sterile drug substance manufacturers
- Draft guidance for industry on process validation: general principies and practices (11/18/2008)
- Draft guidance for industry: submission of documentation in applications for parametric release of human and veterinary drug products terminally sterilized by moist heat processes (8/5/2008)
- International Conference on Harmonisation (ICH); guidance for industry: 03 A impurities in new drug substances (6/5/2008)
- Guidance for industry: container and closure system integrity testing in lieu of sterility testing as a component of the stability protocol for sterile products (2/22/2008)
- International Conference on Harmonisation (ICH); draft guidance: Q10 pharmaceutical quality system (7/12/2007)
- Guidance for industry: quality systems approach to pharmaceutical CGMP regulations (9/29/2006)
- International Conference on Harmonisation (ICH); guidance for industry: 09 quality risk management (6/1/2006)
- International Conference on Harmonisation (ICH); guideline for industry: Q2 A text on validation of analytical procedures (3/1995)
- International Conference on Harmonisation (ICH); guidance for industry: Q28 validation of analytical procedures: methodology (5/19/1997)
- International Conference on Harmonisation (ICH); guidance for industry: Q5E comparability of biotechnological/biological products subject to changes in their manufacturing process (6/29/2005)
Guidance for industry: nonclinical studies for!he safety evaluation of pharmaceutical excipients (5/18/2005) - International Conference on Harmonisation (ICH); guidance for industry: Q1E evaluation of stability data (6/7/2004)
- International Conference on Harmonisation (ICH); guidance for industry: Q1 A(R2) stability testing of new drug substances and products (11/20/2003)
- International Conference on Harmonisation (ICH); guidance for industry: Q1D bracketing and matrixing designs for stability testing of new drug substances and products (1/15/2003)
- Guidance for industry: container closure systems for packaging human drugs and biologics; questions and answers (5/13/2002)
GMP practices are enforced by government regulatory bodies such as the FDA of the United States and the European Medicines Agency (EMEA) of the European Union. GMPs are enforced in the United States by the FDA under the 1938 Food, Drug, and Cosmetic Act. Continued violation of GMP standards in the United States can be prosecuted in courts of law. The World Health Organization (WHO) also has a set of GMP regulations used by pharmaceutical regulators and the pharmaceutical industry in over a hundred countries. However, the European Union and FDA enforce more compliance requirements than the WHO. As time has passed and companies have expanded their international reach, the requirements for GMP compliance worldwide have merged to essentially the same regardless of the manufacturing location.

What are current good manufacturing practices (CGMPs)?
GMP regulations were first proposed in 1963 and enacted into law in 1978 with relatively minor changes. CGMP stands for current good manufacturing practices. The current quality of CGMP covers both any regulatory changes and any changes to the application of GMPs as the industry changes.
The primary areas of change for CGMP regulations include:
- Scientific and technological advances: Examples include computer systems, automated process control, paperless manufacturing, electronic signatures, barrier isolation technologies, biotechnology medicine manufacturing, etc.
- Adverse events: Examples include product tampering incidents, product recalls, contamination incidents, and needle safety precautions.
- Inspection activities and findings: Examples include the 1980’s generic drug scandal, poor GMP compliance in active pharmaceutical ingredient manufacturing, poor documentation practices, lack of aseptic process validation, nonexistent validation studies, etc.
- Industry practice: Examples include the introduction of laboratory management systems, improvements in manufacturing (equipment advances, automation, inspection, etc.), new drug delivery systems (such as liposomal and nanotechnologies), new analytical methods, etc.
What are the most common GMP non-compliance issues found during inspections?
The two most common problems found during GMP inspections are a failure to follow written standard operating procedures (SOPs) and follow good documentation practices.
What are the latest GMP revisions and regulatory standards?
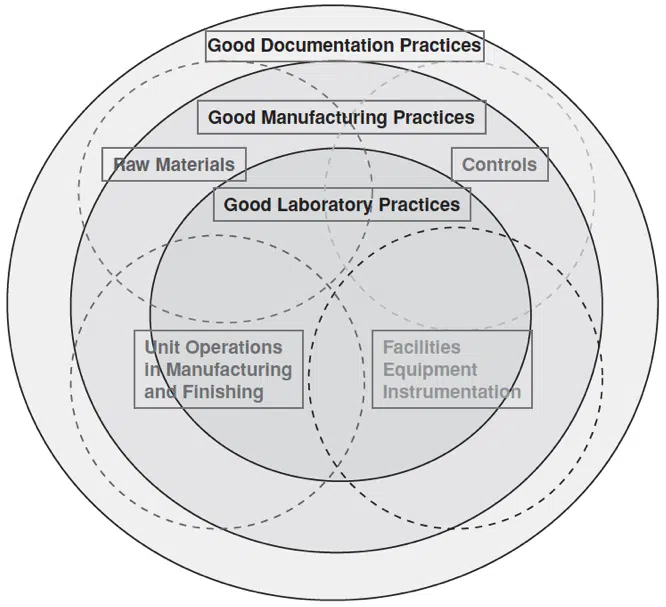
GMPs are constantly evolving. Additionally, the overall focus and philosophy of GMP compliance have changed over the years. The focus was on the product in the earliest years of GMP compliance (the 1960s and 1970s). In the 1980s and 1990s, GMP regulations focused on the process. Now, GMP attention is on quality systems (Fig. 25-1). In 2008, GMP regulations were updated to harmonize the U.S. and international good manufacturing practices.
The main changes to GMP regulations in 2008 were:
- The Environmental Protection Agency (EPA) water standard was eliminated and replaced with the requirement that all water be “safe for human consumption.”
- The requirement to make equipment sterilization clear and in writing.
- The requirement to eliminate asbestos-containing filters.
- The requirement that depyrogenization of sterile containers should be validated (previously, this was an industry practice but not a requirement).
- The requirement to add bioburden testing as an example of in-process testing.
- The requirement to validate procedures to prevent microbiological contamination of all aseptic processes (not just sterilization processes.
- An exception to the requirement for a second personnel check if an automated system is used. Thus, one person can verify that the operations are performed accurately using an automated system.
Summary
Overall, GMP practices are necessary to approve a drug or product for human use in the U.S. and other countries. Compliance with GMPs ensures that products taken by or administered to humans and animals meet certain safety, identity, strength, purity, and quality requirements. GMP practices have evolved, focusing first on the product and, now, on the quality systems implemented to create the product. cGMP is a term used to denote current GMP practices from all GMP practices. Ensure you choose a contract testing organization that can support you with tests for your unique medical device or product needs that meet GMP and ISO standards.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Sharing this in your social netwroks