How To Determine A Disinfectant’s Activity For Cleaning Validations

What is a disinfectant?
A disinfectant is an agent (chemical or physical) that kills or removes harmful microorganisms when applied to a surface. Medical device cleaning validations (FDA cleaning validations) use disinfectant products and disinfectant sprays. Thus, disinfectant activity and the meaning of a disinfectant (disinfectant meaning) is critical to understand for cleaning validations. In contrast, sterilization cycle efficacy and the meaning of sterile is crucial to know for sterilization validations.
What are disinfectants used for, and why are they important?
Validated cleaning and sanitization processes are needed for cleanrooms and other controlled manufacturing environments to prevent microbial contamination of medical and pharmaceutical products. Microbial contamination can occur in various ways during device and drug manufacture. Primary sources of microbial contamination risk include raw ingredient sources, process water, packaging components, processing equipment, and manufacturing operators. Thus, appropriate environmental monitoring that follows current Good Manufacturing Practice (cGMP) is vital to keeping potential contamination risk for health care products and devices low. GMP cleaning and sanitization programs apply to both sterile and nonsterile products.
Disinfectant products and disinfectant sprays are used to keep manufacturing environment surfaces free from harmful contaminants. Disinfectant products and disinfectant sprays may be used on floors, walls, and ceilings. Each disinfectant has a certain bactericidal (bacteria-killing), fungicidal (fungi-killing), and sporicidal (bacterial spore-killing) efficacy. Agents that kill bacteria and spores are particularly good at preventing large-scale bacterial growths known as biofilms. Disinfectant selection is based on the bacterial, fungi, and spore risk of the manufacturing area where the disinfectant will be used. In addition to its microbe-killing profile, a disinfectant should keep the drug product or device under manufacture in mind and should not be harmful to the drug chemistry, drug toxicity, or device materials. Disinfectants (disinfectants meaning sprays, liquids, and other products) are inherently toxic, so drug products must be protected from exposure to most disinfectants used.
What types of chemical disinfectants are available?
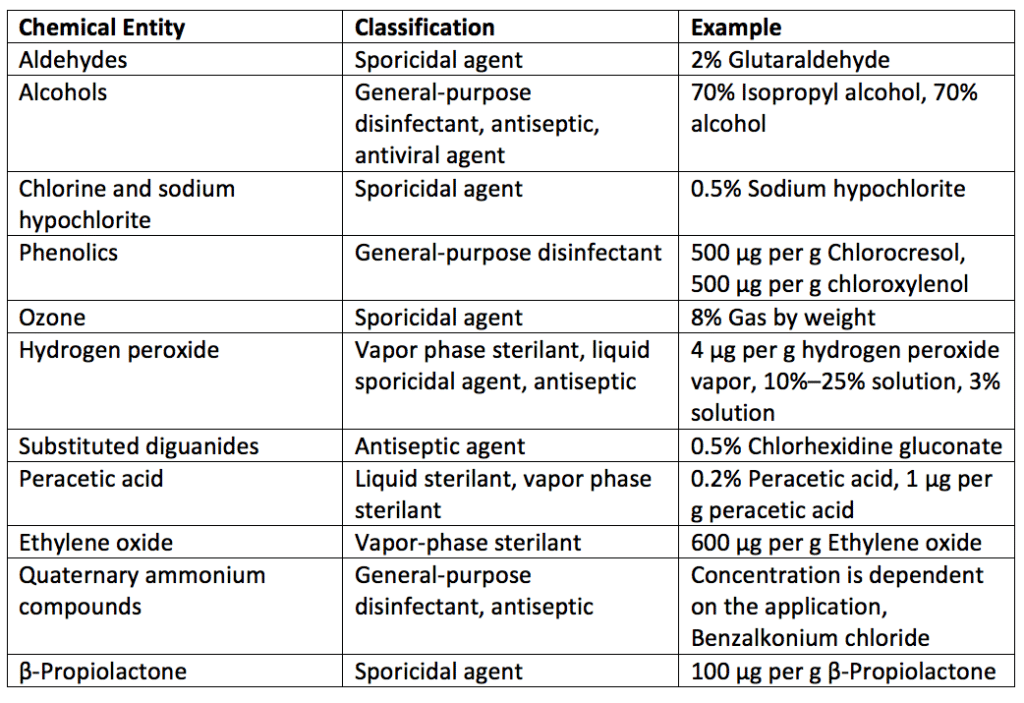
Chemical disinfectants (chemical disinfectants meaning liquids, powders, and gaseous agents) are labeled by their chemical type. Examples of disinfectant chemical families are listed in Table 1. Note that examples and ratios of chemicals used as disinfectants are also detailed in Table 1. For ratios of chemicals, “μg per g” is microgram per gram.
A disinfectant’s effectiveness depends on its natural biocidal activity. Additionally, a disinfectant spray’s ability to kill microbes depends on its concentration, contact time with the surface, the type of surface disinfected, the hardness of the water used for dilution, the quantity of organic material present on the surface, the types of microbes present, and the number of microorganisms populating the surface. Due to the various factors that can influence how well a disinfectant agent works, the Environmental Protection Agency (EPA) registers chemical disinfectants marketed in the United States and requires manufacturers to provide use dilution and required contact time to kill microbes of interest. Some liquid or gas chemical sterilizers used on critical or semi-critical medical devices are defined and regulated by the United States Food and Drug Administration (FDA). One of these chemical sterilizers is ethylene oxide.
How is disinfectant activity determined?
Suppose you plot the log of the number of microorganisms per milliliter surviving in a disinfectant solution. In that case, you will find that first-order kinetics can be applied as an approximation to the reduction in microbe count with respect to time. Note that actual plots of microorganisms per milliliter will show a more sigmoid curve with a slower initial reduction in numbers followed by an increasing microbial reduction rate with respect to disinfectant contact time.
The rate constant, K, for the disinfection process can be calculated with the formula:
K = (1/t)(log N0/N)
Where:
t = the time, in minutes, for the microbial count to be reduced from N0 to N
N0 = the initial number of organisms in colony-forming units (cfu) per milliliter (mL)
N = the final number, in cfu per mL, of organisms
Considering temperature with a first-order chemical reaction, the same concentration of disinfectant will kill organisms more rapidly at elevated temperatures.
This first-order temperature relationship can be expressed as a temperature, T, coefficient per 10°C rise in temperature, Q10, calculated by the formula:
Q10 = Time to decontamination at T°/Time to decontamination at T
Where:
T is T° minus ten.
Ultimately, a first-order reaction is an inadequate description of disinfection. Thus Q10 values are only approximations for an understanding of the effect of disinfectant concentration on microbial reduction.
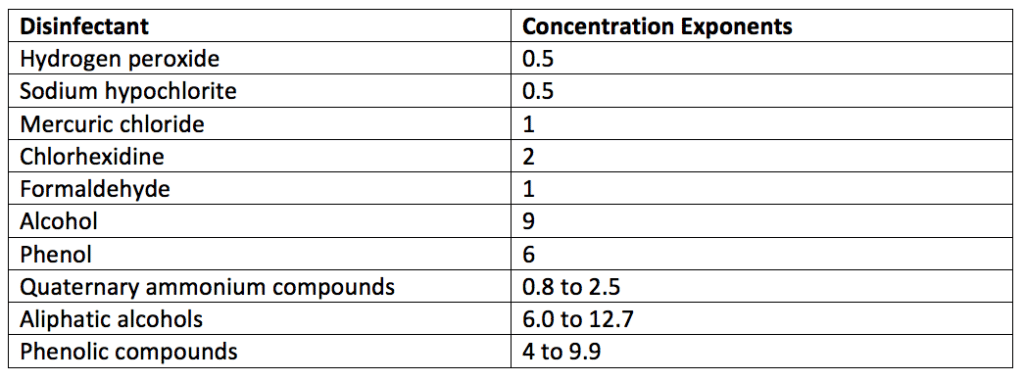
If you make a plot of the log of the time to reduce a microbial population from a standard culture to zero against the log of the disinfectant concentration, you will get a straight line. The slope of this straight line is a concentration exponent, n.
The relationship can be expressed as follows:
n = (log of the kill time at concentration C2) – (log of the kill time at concentration C1)/(log C1 – log C2)
Where:
C1 and C2 are the higher and lower disinfectant concentrations, respectively.
There is a wide difference in the concentration exponent, n, for each disinfectant (disinfectant meaning spray, wipes, liquids, and other products). The concentration exponents for some disinfectants are listed in Table 2. Disinfectants with a larger concentration exponent or dilution coefficient rapidly lose activity when diluted.
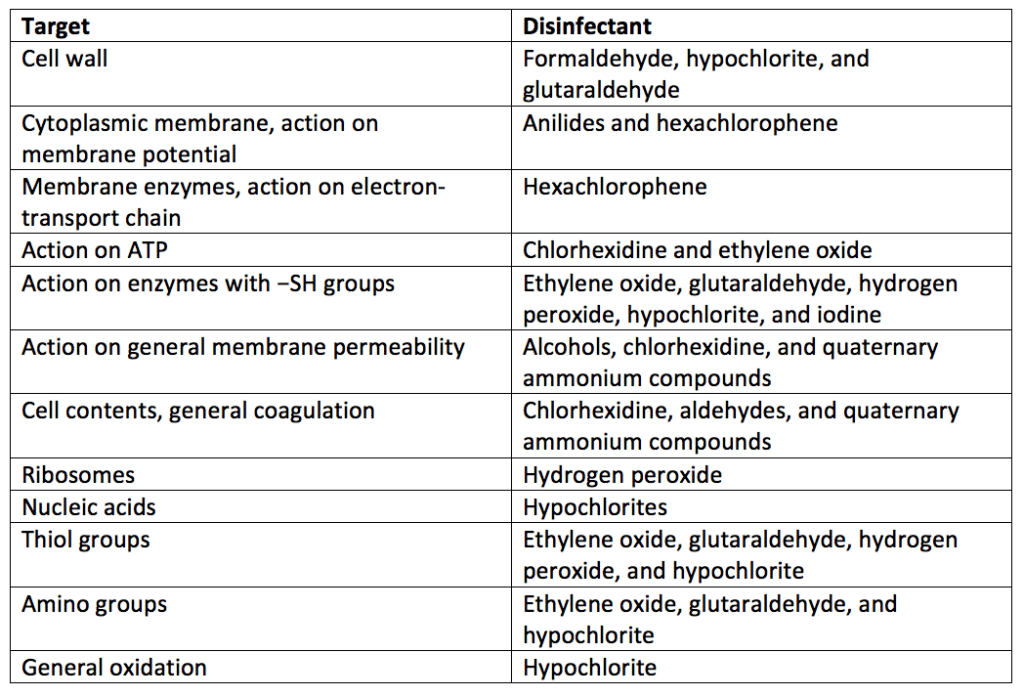
Many disinfectants are more active in either an ionized or nonionized form. The degree of ionization of a disinfectant will depend on the pKa of the agent and the pH of the disinfection environment. For example, with a pKa of ten, phenol will be more effective at a pH below seven where it is nonionized. In addition to overall disinfectant activity, disinfectants work through different mechanisms to kill microbes. Table 3 lists the modes of action of some representative disinfectants.
Summary
Overall, validated cleaning processes are needed for cleanrooms and other controlled manufacturing environments to prevent microbial contamination of medical devices, products, and drugs. Disinfectants are used to keep manufacturing environment surfaces free from harmful contaminants. Each disinfectant has a certain bactericidal (bacteria-killing), fungicidal (fungi-killing), and sporicidal (bacterial spore-killing) efficacy. The activity of a disinfectant agent against various microbes can be approximated using first-order kinetics. The effectiveness of the disinfectant will increase as the temperature rises. Concentration exponents for disinfectants can be calculated using the log of the kill time at two concentrations. All in all, ensure you choose a contract testing organization that can support you with appropriate cleaning validations for your unique medical device or product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1072> Disinfectants And Antiseptics. Rockville, MD, USA. 2021. (USP <1072>).
Sharing this in your social netwroks

