Requirements You Must Meet For FDA-Approved, Medical Face Masks
What does ASTM F2100 cover?
Face masks are essential for protecting the public and healthcare workers from the viruses and other illnesses they are exposed to during their day-to-day activities. ASTM F2100 covers the requirements for face mask materials used in healthcare services such as surgery and patient care. Medical face mask performance meets three levels of criteria. These criteria are determined based on testing face mask materials for bacterial filtration efficiency, differential pressure, sub-micron particulate filtration efficiency, resistance to penetration by synthetic blood, and flammability. ASTM F2100 does not cover mask design requirements, which can impact the effectiveness of the medical face mask barrier and breathability properties.
Further, ASTM only evaluates the materials used in face masks, not the seal of the medical face mask against the wearer’s face. Thus, the requirements necessary for regulated respiratory protection (such as NIOSH respirators) are not covered. Also, ASTM F2100 does not cover the facial fit of the mask and material degradation due to the wearer (perspiration, talking, sneezing, and length of mask use).
Often, one material property for a mask comes at the expense of another. For example, improved resistance of face mask material to synthetic blood penetration can reduce face mask breathability. Generally, improving synthetic blood penetration resistance, bacterial filtration efficiency, and sub-micron particulate filtration efficiency will reduce the fabric’s breathability. Though design features are not covered in ASTM F2100, selecting an appropriate face mask material classification for the exposure hazard risk is crucial. General-use masks, for example, provide minimal fluid penetration resistance and are best for tasks with minimal liquid exposure to the wearer. Where healthcare tasks involve exposure to sub-micron particles, such as laser or electrocautery surgery, sub-micron filtering masks will be needed. Sub-micron particle exposure parameters would also apply to masks designed to protect against viral illnesses. Fluid-resistant medical faces masks are best for exposure to blood or bodily fluids (such as during surgical procedures). All in all, when designing or purchasing a face mask, ensure that the material properties of the mask meet the requirement for intended mask use.
What are the ASTM F2100 classifications and requirements for medical face masks?
Classifications
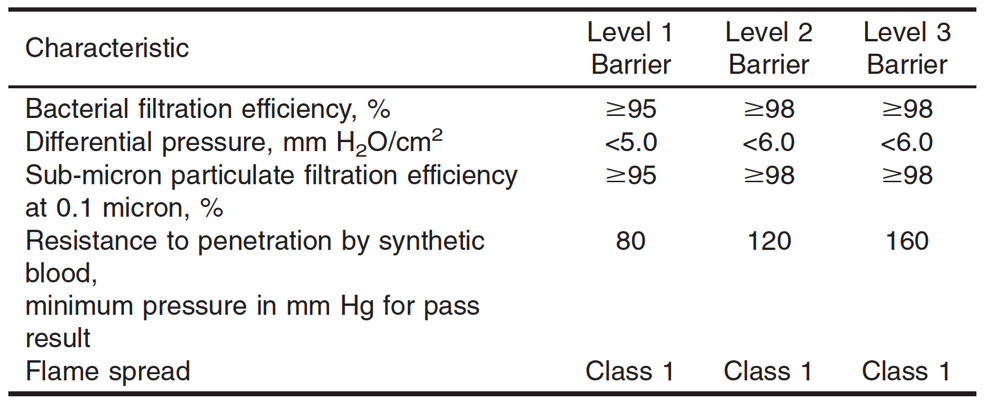
Medical face mask performance classes are based on the barrier performance properties of the medical face mask materials. There are three levels of face mask classification for healthcare (Level 1, Level 2, and Level 3). Specific face mask material requirements for Level 1- Level 3 masks are given in Table 1 (reproduced from ASTM 2100) below.
Requirements
Simply put, the properties of the medical face mask material met the specifications detailed in Table 1. At a minimum, materials used in the construction of medical face masks will meet the requirements for Level 1. The tests used to evaluate the properties of the face mask materials are bacterial filtration efficiency, differential pressure, sub-micron particulate filtration, resistance to penetration by synthetic blood, and flammability. Bacterial filtration efficiency assesses how well a mask material protects the wearer from bacterial exposure. Differential pressure estimates the resistance a material has to breathing to measure differential pressure per unit area. Sub-micron particulate filtration determines how well a material can protect the wearer from different-sized particles. Whereas resistance to penetration by synthetic blood determines the approximate protection a mask material will give if exposed to blood. Flammability requirements are the resistance of a material to incineration upon heat exposure. Flammability requirements are the same for all mask classifications.

Summary
Coming out of a global pandemic, facial mask manufacturing is at an all-time high. ASTM F2100 covers the basic requirements for three classes of medical masks (Level 1, Level 2, and Level 3). These classifications are based on mask material performance when assessed for bacterial filtration efficiency, differential pressure, sub-micron particulate filtration efficiency, resistance to penetration by synthetic blood, and flammability. If outsourcing the evaluation of your facial mask materials, ensure you choose a contract testing organization that can support your needs.
MycoScience is a contract manufacturing organization specializing in a variety of ASTM regulated tests. MycoScience is a contract manufacturing organization that specializes in filling sterile syringes and vials for parenteral products. MycoScience also offers Bacterial Endotoxin Testing, Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services for medical device companies, and allied industries. MycoScience is an ISO 13485 certified facility.
References
American Society for Testing and Materials. Standard Specification for Performance of Materials Used in Medical Face Masks. West Conshohocken, PA, United States. (ASTM F2100-19).
Sharing this in your social netwroks

