Microbial Aerosol Challenge Vs. Disinfectant Challenge Testing For Parenteral Products
What is a microbial challenge?
The “microbial” in microbial challenge testing refers to microorganisms. The “challenge” component of microbial challenge testing refers to exposure. In a physical challenge, you are exposing your body to physical challenges. In a microbial challenge, a product, agent, or process is exposed to (challenged by) microbes. Microbial aerosol testing for drug vials utilize microbial challenges to ensure that products are able to maintain their sterility over the lifetime of the product. Microbial challenges may also be used by a disinfectant testing laboratory to evaluate manufacturing disinfectants.
What is microbial aerosol testing?
Microbial aerosol challenge testing is used to test the enclosures of filled product vials (like drug vials) to ensure that the vial enclosure does not allow microbes to enter following the entry and exit of syringe needles during dosage draws.
Why is microbial aerosol testing important?
Microbial aerosol challenge testing is essential for verifying multi-use drug vial enclosure systems (such as those used for vaccines and other parenteral products). Microbial aerosol challenge testing ensures that the sterile or aseptic drug vials act as an effective microbial barrier and preserve the product’s sterility even when the outside of the drug vial is exposed to high concentrations of bacteria.
What is a disinfectant?
A disinfectant is an agent (chemical or physical) that kills or removes harmful microorganisms when applied to a surface. A disinfectant’s effectiveness can be evaluated by disinfectant testing laboratories and depends on the disinfectant’s natural biocidal activity.
A disinfectant’s ability to kill microbes depends on:
- Concentration
- Contact time with the surface
- Surface type disinfected
- The hardness of the water used for dilution
- Quantity of organic material present on the surface
- Microbial types present
- Number of microorganisms populating the surface
What are disinfectants used for, and why are they important?
Validated cleaning and sanitization processes are needed for cleanrooms and other controlled manufacturing environments to prevent microbial contamination of medical and pharmaceutical products. Microbial contamination can occur in various ways during device and drug manufacture. Primary sources of microbial contamination risk include raw ingredient sources, process water, packaging components, processing equipment, and manufacturing operators. Thus, appropriate environmental monitoring that follows current Good Manufacturing Practice (cGMP) is vital to keeping potential contamination risk for health care products and devices low. GMP cleaning and sanitization programs apply to both sterile and nonsterile products.
Disinfectants are used to keep manufacturing environment surfaces free from harmful contaminants. Disinfectants may be used on floors, walls, and ceilings. Each disinfectant has a certain bacteria-killing (bactericidal), fungi-killing (fungicidal), and sporicidal efficacy. Agents that kill bacteria and spores are particularly good at preventing large-scale bacterial growths known as biofilms. Disinfectant selection is based on the manufacturing area’s bacterial, fungi, and spore risk where the disinfectant will be used. Disinfectant testing laboratories support manufacturers in evaluating disinfectant efficacy. In addition to its microbe-killing profile, a disinfectant should keep the drug product or device under manufacture in mind and should not be harmful to the drug chemistry, drug toxicity, or device materials. Disinfectants are inherently toxic, so disinfectant testing laboratories must take care when evaluating disinfectant agents. Further, drug products must be protected from exposure to most disinfectants used.
How is microbial aerosol testing performed?
Microbial aerosol challenge testing evaluates a finished product’s package integrity by exposing the terminally sterilized, packaged product (such as a drug vial) to aerosolized Bacillus atrophaeus bacteria. After the package exterior has been exposed to Bacillus atrophaeus, the package contents are tested for bacterial contamination.
Microbial aerosol challenge testing begins with the preparation of Trypticase Soy Broth (TSB) media that will be used as a negative control (without bacteria) and positive control (with bacteria). All microbial aerosol challenge methods are performed in an ISO Class 5 laminar flow hood to maintain sterility during the assessment. All positive controls, negative controls, and samples will have adapters for syringe use. For the preconditioning process, ten samples (terminally sterilized vials containing product) and all controls are held statically in an ISO Class 7 controlled environment for 28 days. Five samples are laid down on their side, and five are left standing on their base during the 28 days of storage. Two dose removals will be performed from the TSB vial at times of 0, 7, 14, 21, and 28 days. After 28 days of sample conditioning, the vial adapter/syringe assembly will undergo the microbial aerosol challenge test.
A Bacillus atrophaeus suspension is prepared for the microbial challenge so that the aerosol chamber receives a minimum of 1.0 x 107 spores when the bacterial is aerosolized. The final suspension concentration is verified using the spread plate method. Once the aerosol chamber with Bacillus atrophaeus suspension has been prepared, the ten test media sample vials with attached syringe-adapter assemblies are loaded, equally spaced and upright, into the aerosol chamber along with the positive controls. Positive controls are created by piercing the vial septum with a sterile 23-gauge needle and leaving the needle in place during the microbial challenge. Negative control vials are not loaded into the chamber. Once all positive controls and sample vials are loaded into the aerosol chamber, the doors are sealed, and the chamber fan is turned on. The prepared Bacillus atrophaeus suspension is loaded to the chamber’s nebulizers, and the suspension is aerosolized until the nebulizers are empty. Samples are left in the chamber for an hour after the suspension has been fully aerosolized. Next, samples are taken out of the chamber, and the exterior is thoroughly disinfected. Finally, all positive control, negative control, and test drug vials are incubated upright for seven days.
After seven days, all control and sample vials are checked for microbial growth. The level of microbial growth is assessed by streaking the samples onto TSA agar and incubating them. Any growth on the agar plates is compared to Bacillus atrophaeus by Gram staining and direct microscopic observation. Negative TSB vial sterility results indicate that the sterile barrier vial system acted as an effective microbial barrier for the product under the microbial aerosol challenge testing conditions. Positive TSB media vial results indicate that the sample closures were not an effective microbial barrier.
How is disinfectant challenge testing performed?
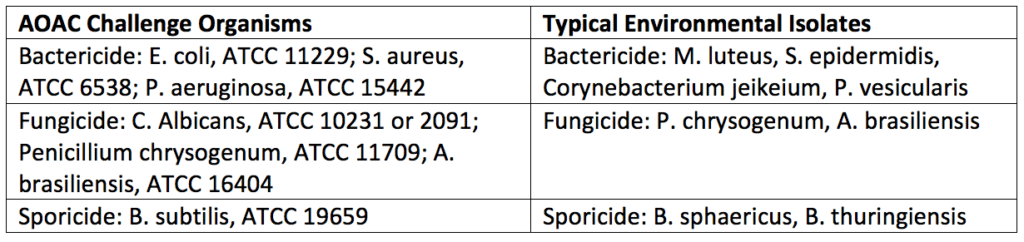
EPA validated disinfectants, and their directions, are not sufficient for use in GMP manufacturing for pharmaceutical, biotechnological, and medical device industries. Thus, official disinfectant testing (in some cases by a disinfectant testing laboratory) must be performed for disinfectants used in manufacturing environments so that these disinfectants meet aseptic and non-aseptic manufacturing cleaning validation criteria. In the United States, use-dilution tests and surface challenge tests are completed for disinfectant challenges. Use-dilution tests are used by disinfectant testing laboratories to screen disinfectants for their efficacy (at various concentrations and contact times) against a wide range of standard test organisms and environmental isolates. In contrast, surface challenge tests use microorganisms based on typical environmental isolates (for the microbial challenge) and apply disinfectants to surfaces at predetermined concentrations with a specified contact time.
After the surface challenge and use-dilution testing has been performed by a disinfectant testing laboratory technician, a statistical comparison (log reduction) of the numbers of microorganisms isolated before and after disinfection would be completed. Test organisms are counted using swabs, surface rinse, or contact plate methods. As a control, neutralizers that inactivate the disinfectants should be included in either the diluent or microbiological media used for microbe counting. For acceptance criteria, enough organisms need to be killed or removed on a 2-inch × 2-inch square of the surface being decontaminated. Generally, a 2-log reduction (for bacterial spores) or a 3-log reduction (for vegetative bacteria) will need to be accomplished during a predetermined contact time (i.e., 10 minutes). Disinfectants are less effective against the higher numbers of microorganisms used in challenge tests and more effective against the numbers found in clean rooms. Further, the organisms typically used in laboratory tests are more resistant (except for spores) than those used in laboratory tests found in an actual manufacturing environment. Thus, disinfectant challenge tests are robust, and disinfectants that pass these criteria will be more than capable of disinfecting the actual manufacturing area they are selected to maintain. Typical challenge organisms used for disinfectant testing are listed in Table 1 below.
How is sterilization by filtration performed?
Simply speaking, sterilization by filtration is performed by flowing a non-sterile product through a sterile filter. The sterile filter then removes particulate matter and microorganisms from the non-sterile liquid formulation. In eliminating particulate matter and microorganisms, the filter sterilizes the product. Filters sterilize through a combination of sieving, screening, entrapment, impaction, and electrostatic attraction of particles (including microbes). Particles are collected on the surface of the filter during sieving and screening. In contrast, entrapment occurs when particles smaller than filter pores lodge themselves within the filter’s passageway. Electrostatic attraction absorbs particles of opposite charge to the filter surface. Note that entrapped particles, or particles held with an electrostatic charge, can be dislodged and end up in the product filtrate when flow rates or pressure is increased or varied. Keep this in mind for viscous products.
Membrane filters are used for sterilizing solutions because of their ability to be nonreactive with most products, retain particles, and not shed debris. Products with peptides should use polysulfone and polyvinylidene difluoride filters to prevent accidental protein adsorption to the filter membrane. Often filter membranes are composed of cellulose esters, nylon, polysulfone, polycarbonate, PVDF, or polytetrafluoroethylene. Structurally, filters are designed to increase surface area and thus increase the flow rate. Standard filter designs are flat membranes, pleated cylinders, and cartridge structures. During use, the product enters the outside of the filter cartridge with applied positive pressure forcing the fluid inward through the filter. The sterile filtrate then exits from the center of the cartridge. The filter and housing are steam sterilized before product filtration, typically by steam-in-place (SIP) systems. Pressurization during SIP sterilization must be gradual to maintain filter integrity. Often filters are dried with compressed gas after sterilization and before use.
For sterile filtration, the product must not adversely affect the particle retention of the filter. Also, the product must not cause the filter to leach any materials into the product. Filter manufacturers provide information on the liquid volume a filter can flush before oxidizable substances are released. Further, filter manufacturers provide data on extractables obtained with exposure to various solvents. Common filter extractables include oligomers, mold release agents, antioxidants, wetting agents, manufacturing debris, plasticizers, and 0-ring material. Sometimes protein biologics will bind to the filter material. In this case, a pre-flush step may be used before filtration to occupy any available binding sites for the proteins and remove any potential extractables.
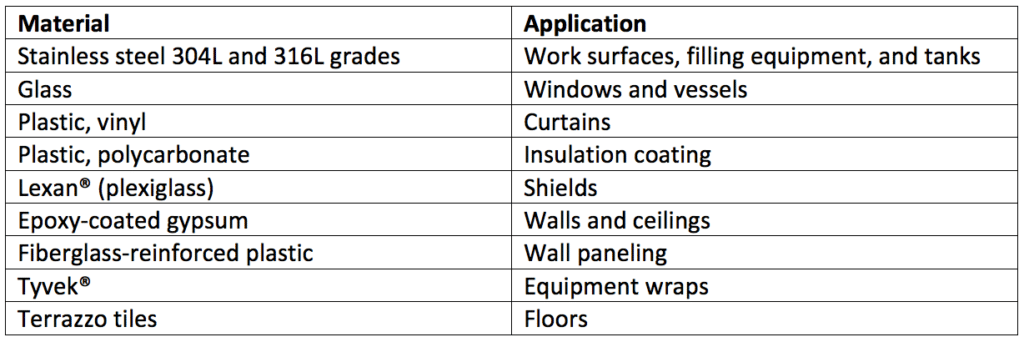
Cleanroom construction isn’t uniform. Thus, each material within the cleanroom and other controlled areas must be evaluated separately to validate the efficacy of a given disinfectant used on its surface. Table 2 is a list of common materials used in cleanroom construction.
What are the similarities and differences between microbial aerosol challenge and disinfectant challenge testing?
Microbial aerosol challenges use a single bacteria type (Bacillus atrophaeus) to evaluate the integrity of drug vial elastomeric stoppers for multidose products. Microbial aerosol challenges can also be used to test the integrity of other packaged products. In contrast, disinfectant challenge testing uses multiple types of bacteria and fungi to evaluate the effectiveness of a cleaning agent at removing and preventing microbial growth on surfaces. Microbial counting methods for the microbial aerosol challenge and disinfectant challenge testing are similar. Overall, the aim of microbial aerosol challenge and disinfectant challenge testing and the microbes used for each challenge is different despite similarities in how quantitative microbial counts are collected.
Summary
Overall, both microbial aerosol challenge and disinfectant challenge testing are imperative for regulatory approval of parenteral product package integrity and manufacturing sterility, respectively. These tests ensure that parenteral products, manufacturing environments, and packaging are sterile. Products that are sterile ensure that patients will not be at risk of illness following product use. Disinfectant challenge testing, in particular, keeps manufacturing environment surfaces free from harmful contaminants and is a part of GMP-cleaning validations. In contrast, microbial aerosol challenge testing is critical for package integrity testing for multidose parenteral products. All in all, ensure you choose a contract manufacturing organization that can support you with appropriate microbial aerosol challenge testing and cleaning validations for your unique cosmetic or parenteral product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
ANSI/AAMI/ISO 11607-1, 2006/A1:2014, Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging, Amendment 1.
ASTM F1608 – 16, Standard Test Method for Microbial Ranking of Porous Packaging Materials (Exposure Chamber Method).
Technical Report No. 27, Pharmaceutical Packaging integrity, 1998, PDA.
Technical Information Bulletin No. 4, Aspects of Container/closure Integrity, PDA.
United States Pharmacopeial Convention. <1072> Disinfectants And Antiseptics. Rockville, MD, USA. 2021. (USP <1072>).
Sharing this in your social netwroks

