Preservative Efficacy Testing And Cytotoxicity Testing Principles And Differences
What are preservatives?
Preservatives are substances mixed into creams, gels, and other liquids. Preservatives are used to prevent the growth of bacteria, fungi, and other microorganisms within a medical, cosmetic, household, or food product. Preservatives also help keep the freshness of the appearance of a product and keep its consistency intact over time.
What is preservative efficacy testing (PET)?
Preservative efficacy testing, also known as preservative challenge testing, determines the effectiveness of a preservative during its shelf life and evaluates how well a product withstands microbial contamination during use.
What is cytotoxicity?
Cytotoxicity refers to molecules and compounds that are poisonous to living cells. Cytotoxins are often chemical but can also be from natural or biological sources.
What is cytotoxicity testing?
Cytotoxicity testing evaluates the biological reactivity of mammalian cells and tissues to contact with elastomeric plastics, excipients, and other materials that will come in direct or indirect patient contact during medical product use. Cytotoxicity is significant as it evaluates the biological effects of a sample’s leachable chemicals. The types of cytotoxicity testing to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within.
What products require preservative efficacy testing?
Preservative efficacy testing can be used to determine the best preservative to use in your topical formulation and the minimum effective concentration needed to preserve the pharmaceutical, food, or biotechnology product being assessed. Topical formulations (gels, creams, ointments, and lotions) comprise a water or oil base that delivers various medications, natural substances (such as herbs), or moisture through the skin. Topical cosmetics contain preservatives to support the stability of the formulation and prevent microbial growth during repeated product use. Preservative challenge testing is also used for parenteral products containing multiple doses. In these products, antimicrobial preservatives inhibit the growth of any microorganisms introduced during repeated insertion and withdrawal to load individual amounts.
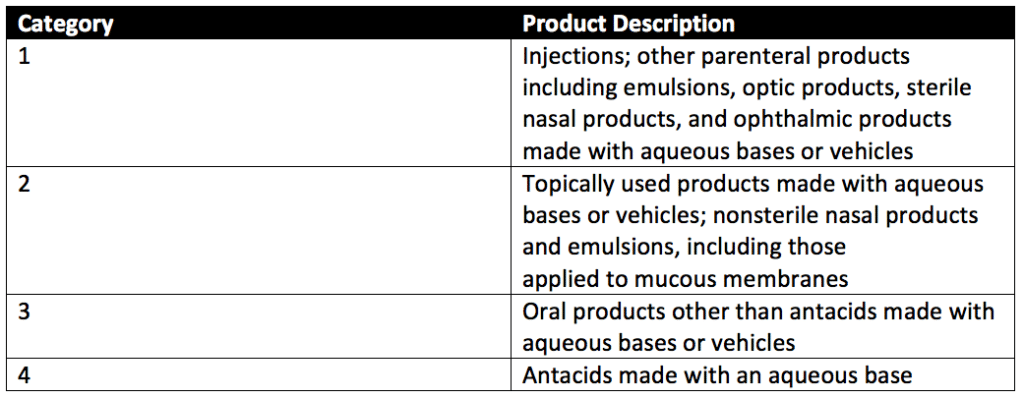
Preservative efficacy testing can evaluate four categories of products with preservative challenge testing. These product categories are detailed in Table 1 of USP 51, reproduced below.
What products require cytotoxicity testing?
Cytotoxicity testing is especially for medical products or devices that will be inserted into the human body. Cytotoxicity testing protects patients from exposure to cytotoxins on or within medical devices or products. By doing so, cytotoxicity testing prevents illness or organ failure resulting from leachable toxins. Thus, cytotoxicity testing is an important quality control step for medical devices, parenteral products, and other medical products.
How is preservative challenge testing performed?
Three approaches are used in preservative challenge testing. These approaches come from the Schülke KoKo Test, United States Pharmacopeia (USP), and the European Pharmacopeia.
Schülke KoKo Test vs. U.S. Pharmacopeia vs. European Pharmacopeia
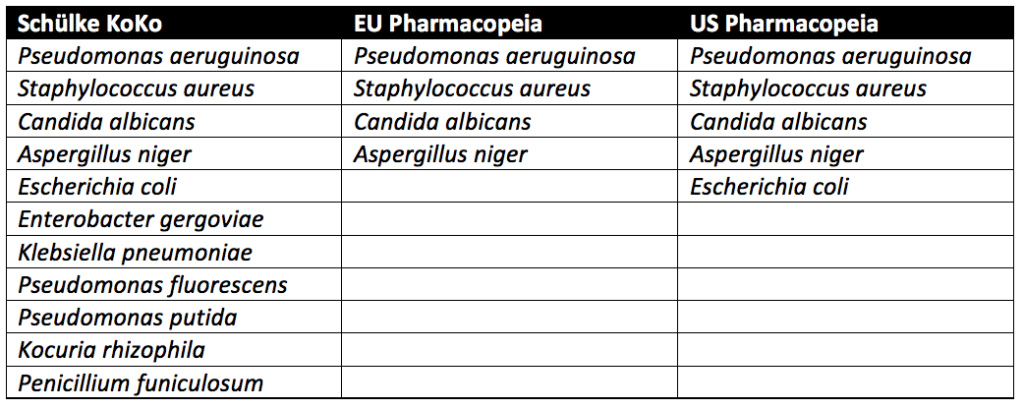
Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus niger, and Candida albicans must be tested for in all cosmetic products sold in the European Union. In addition to the microbes above, testing with microbes known to lead to spoilage of cosmetic products is recommended but not required. In the United States, the USP guidelines require testing of Escherichia coli in addition to Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus niger, and Candida albicans. Spoilage microbes are not needed for PET. In contrast to the pharmacopeia tests, which only evaluate pathogenic microbes, the Schülke KoKo test evaluates product spoiling microorganisms. Schülke KoKo test’s spoiling microorganisms are based on decades of cosmetic testing experience from Schülke & Mayr in Germany.
USP preservative efficacy testing evaluates cosmetic products by exposing them to a single microbial strain at a time. Additional details on USP preservative challenge testing, which follows similar procedures to the European Pharmacopeia, can be found through reading our articles on Preservative Challenge Testing And USP 51 and Preservative Efficacy Testing For Medical Devices. In contrast, for the Schülke KoKo test, the single cultivated microbes are brought together into a mixed suspension to challenge the cosmetic product. A new mixed suspension is prepared for each of the six inoculation cycles for Schülke KoKo testing. Since the Schülke KoKo test uses a mixed microbe inoculation, it simulates microbial exposure of a product during production, filling, and use. Indeed, a product would likely be exposed to multiple microbes at once instead of one at a time. Overall, though different from the USP and European Pharmacopeia methods, the Schülke KoKo Test is a reliable test method for assessing the efficacy of antimicrobial preservation of cosmetic products.
First, cultures of Candida albicans (ATCC No. 10231), Aspergillus brasiliensis (ATCC No. 16404), Escherichia coli (ATCC No. 8739), Pseudomonas aeruginosa (ATCC No. 9027), and Staphylococcus aureus (ATCC No. 6538) are prepared. Stock cultures of these organisms are prepared by centrifuging an ATCC culture removing residual media from the prior culture and resuspending the microorganisms in a sterile suspension fluid. The suspension fluids for each microorganism referenced above are prepared at a microbial count of about 1 × 108 colony-forming units per milliliter (CFU/mL).
Preservative efficacy testing is performed in five sterile, capped containers. Suppose the product’s original container is sterile, can be entered aseptically, and holds an appropriate product volume. In that case, the original filled product containers may be used. Each of the five product samples is injected with a test suspension of either Candida albicans, Aspergillus brasiliensis, Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus aureus. Each product sample is exposed to only a single microbe of the five listed, and all microbes are exposed to the product during testing. The microbial test suspension injected is between 0.5%- 1% of the volume of the product under assessment and is at a concentration of 1 × 105 and 1 × 106 CFU/ml of the product for category 1-3 products. The final concentration per ml of category 4 products is between 1 × 103 and 1 × 104 CFU/mL.
Each of the five product samples with their respective microbial injections is incubated at a simulated room temperature of 22.5 ± 2.5°C or 32.5 ± 2.5°C depending upon the microbe the product sample is exposed. Microbial counts are taken at 7, 14, and 28 days for most microorganisms tested. The plate-count method determines the number of CFU present in each inoculated product sample. This plate-count method is completed in duplicate. Once all counts are taken, the change in log10 of the concentration of CFU/ml is calculated for each microorganism. The requirements for antimicrobial effectiveness are met if no increase in microbial growth for the various organisms tested occurs within the timeframes specific to each organism. are met. No increase in microbial growth is defined as not more than 0.5 log10 units more than the previous microbial growth value.
How are cytotoxicity tests performed?
Cytotoxicity testing is either performed in-vitro, using cell cultures, or in-vivo, using live animals. Most medical devices, parenteral products, and other medical products will only require in-vitro cytotoxicity testing. In-vitro methods of cytotoxicity testing include direct contact, agar diffusion, and elution testing. If in-vitro testing cannot be performed, in-vivo testing is required. In-vivo methods of cytotoxicity testing include intracutaneous injection, systemic, and implantation testing. In-vitro cytotoxicity testing is covered in USP 87, while in-vivo testing is covered in USP 88.
What are the differences between preservative efficacy testing and cytotoxicity testing?
Cytotoxicity testing is used for toxin prevention. In contrast, preservative challenge testing determines the best preservative for use and the minimum effective concentration needed to preserve multidose parenteral products, topicals products, food, or other healthcare items that contain preservatives. Together preservative efficacy and cytotoxicity tests ensure that parenteral products, cosmetic products, and medical devices meet the regulatory safety criteria for toxicity and antimicrobial activity.
Summary
Overall, preservative efficacy testing (PET) and cytotoxicity testing are imperative for the regulatory approval of pharmaceuticals, cosmetics, and medical devices. These tests ensure that parenteral products, topicals, and medical devices are non-toxic and have sufficient antimicrobial activity to keep patients safe during product use. All in all, ensure you choose a contract manufacturing organization that can support you with appropriate cytotoxicity and preservative efficacy testing for your unique cosmetic, pharmaceutical, or medical device product needs.
MycoScience is a contract manufacturing organization specializing in Preservative Efficacy Testing, Cytotoxicity Testing and sterile syringe and vial filling. MycoScience also offers Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <51> Antimicrobial Effectiveness Testing. Rockville, MD, USA. 2021. (USPC <51>).
United States Pharmacopeial Convention. <87> Biological Reactivity Tests, In Vitro. Rockville, MD, USA. 2021. (USPC <87>).
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Sharing this in your social netwroks

