What Is Parametric Release Testing For Medical Devices And Products?
Parametric release requires robust manufacturing process controls that allow a finished product to be immediately released instead of going through end-product testing to establish a product’s safety, purity, sterility, and efficacy. For parametric release to be an option for your product, in-process manufacturing conditions must show that key product quality attributes (acceptance criteria) are attained and maintained throughout relevant manufacturing steps. An example of a key product quality attribute is sterility acceptance criteria. If the in-process controls can prove a certain level of sterility, no end-product sterility testing is necessary. The radiation sterilization of products performed following the International Organization for Standardization (ISO) 11137 is a sterilization process that has been used with in-process controls to replace end-product sterility testing. Well-designed, validated, and controlled parametric release processes (such as sterile product manufacturing systems) have a very low probability of microbial contamination. Indeed, the likelihood of a nonsterile unit (PNSU) is less than one in a million. Parametric release, especially for sterility testing, fulfills the requirements of USP 71. Further, parametric release process controls often exceed USP 71 sterility requirements since all products are passing the parametric release testing requirements compared to a sampling of products being tested for sterility testing as a representative of the entire product batch. Due to the advantages of parametric release, a parametric release program is commonly used for medical product release and should be used instead of sterility testing when feasible. Note that the application of parametric release requires prior regulatory approval. Parametric release to avoid end-product sterility testing must show a PNSU equal to or less than one in a million.
Terminal sterilization processes for the manufacture of sterile products are candidates for parametric release. If the terminal sterilization method consistently achieves a PNSU of equal to or less than one in a million, the requirements for parametric release are met. Terminal sterilization may be conducted using any sterilization method that retains product quality and demonstrates a PNSU of equal to or less than one in a million. Sterilization methods that have been successfully used in parametric release processes to sterilize drug products or medical devices in their primary package are moist heat, dry heat, gas, and radiation.
What are parametric release testing requirements?
Manufacturing organizations wishing to develop and validate parametric release programs must define the user requirements specification (URS) for the product and ensure that all conditions within the URS are met. URS process control validations must be acceptable to meet regulatory authority criteria. The URS should include all critical functions of the medical product or drug, necessary equipment, manufacturing processes, manufacturing environment requirements, monitoring and control requirements, operational requirements, and any other essential characteristics for ensuring product function and sterility. At times it is useful for the URS to be generated by the product user.
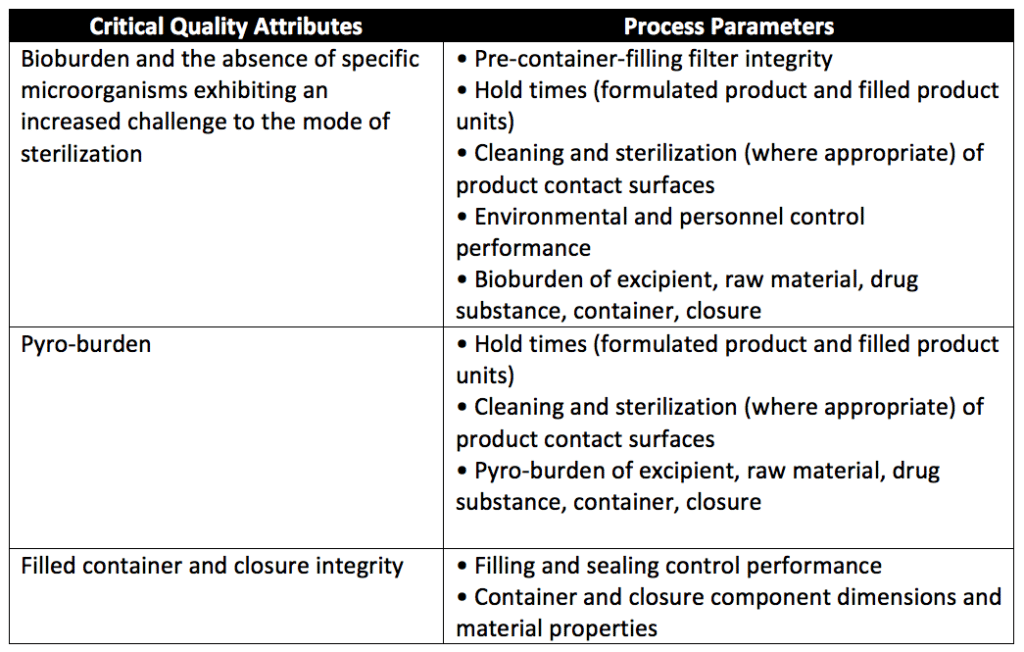
Parametric release quality attributes and process parameters to be included in the URS are:
- Critical quality attributes: Establish criteria for immediately before and immediately after sterilization to ensure that the sterility of every unit is sustained through expiry.
- Process parameters: Establish accurate predictors of product sterility assurance. The operating ranges are developed based on the sterilization process, sterilization process capability, and calibration tolerance limits. Process parameters are based upon the sterilization process’s risk. Control of critical process parameters within their validated operating ranges assures the sterility of products manufactured within the parametric release program. Any products processed where the critical process parameters are not controlled within the established and validated operating ranges will be disposed.
- Pre-sterilization parameters ensure a controlled bioburden exists on products entering a sterilization process. The level of control of the manufacturing environment and pre-sterilization product bioburden (microbe amount, frequency of recovery, and species) should be within validated ranges to ensure the efficacy of the subsequent sterilization process. Historical data for spore recovery should be infrequent. The pyrogen burden should be appropriately controlled throughout the manufacturing process. Table 1 summarizes the minimum pre-sterilization quality attributes and their process parameters.
- Sterilization process parameters: During the sterilization process, critical process parameters are monitored and recorded to ensure a predictable level of product sterilization. Appropriate chemical, physical, or biological load monitors are used to demonstrate the sterility of the load. Load monitors and biological indicators are placed at product locations that are difficult to sterilize. If all product locations are sterilized appropriately following the sterilization process, the sterilization process parameters have been met and ensure product sterility. Load monitors are also placed in specific locations to ensure each unit receives the required minimum sterilizing conditions. In parametric release programs, electronic and automated systems are used to assure control of the sterilization cycle. A computerized assessment of the sterilization cycle data against validated critical process parameters proves that sterilization criteria have been met. Two critical sterilization process parameters are the distribution profile of the physical or chemical sterilization agent throughout the entire cycle and sterilization cycle time duration (minimum and maximum for any phases in the cycle).
How do you perform risk assessments for parametric release programs?
A parametric release program thoroughly assesses and manages risk. All risk assessments include the process parameters, critical process parameters, and the means of mitigating the risk to achieving critical quality attributes. For new products or sterilization processes, a risk assessment is conducted during process development. For existing products or processes, the risk assessments include historical data.
The following risk assessments are necessary for a parametric release program:
- Pre-sterilization product bioburden: Assess the risk of high bioburden or highly resistant microorganisms (e.g., spore formers) during processing. Assessment includes the means to count the microbes, characterize the microbes, and evaluate opportunities for and ways that product contamination could occur. The risk assessment should also include pyrogen risk and any risk pyrogens would have to the product’s functionality if the product were exposed to them.
- Loading patterns: Evaluate the positioning, stacking, and distribution of product units within the sterilizer. This includes the potential loading damage or damage of products during sterilization for each sanitation process. Loading pattern evaluations also include the risk of product units failing to achieve sterility due to positioning, stacking, loading, or the sterilization process itself.
- Container and closure: Evaluate the container-closure system for the risk of integrity breaches before, during, and after sterilization. The container-closure system evaluation.
- Product segregation: Risk assessment of products after sterilization should evaluate the means of physical segregation (manual or automated) of sterilized products from pre-sterilized and non-sterilized products.
Summary
Overall, parametric release requires robust manufacturing process controls that allow a finished product to be immediately released instead of going through end-product testing to establish sterility. For parametric release to be an option for your product, in-process manufacturing conditions must show that key product quality attributes (acceptance criteria) are attained and maintained throughout relevant manufacturing steps. Due to the advantages of parametric release, parametric release programs are commonly used for medical product release and should be used instead of sterility testing when feasible. Note that the application of parametric release requires prior regulatory approval. Parametric release to avoid end-product sterility testing must show a PNSU of equal to or less than one in a million. If you wish to use a parametric release program for your medical product, ensure you choose a contract manufacturing organization that can support you with appropriate process validations for your unique medical device or product needs.
MycoScience is a contract manufacturing organization that specializes in filling sterile syringes and vials for parenteral products. MycoScience also offers Sterilization Validations, Bacterial Endotoxin Testing, Preservative Efficacy Testing, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services for medical device companies, and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1222> Terminally Sterilized Pharmaceutical Products- Parametric Release. Rockville, MD, USA. 2021. (USPC <1222>).
International Organization for Standardization. ISO 15882:2008: Sterilization of health care products—Chemical indicators—Guidance for selection, use and interpretation of results.
Sharing this in your social netwroks

