How To Select Water For Use In Cosmetic & Pharmaceutical Products
Why is the environmental monitoring of water essential for sterile products?
Microbes in the water can easily contaminate sterile products during manufacturing. Thus, knowledge of the microbiome within a water system is critical to monitor and control the microbial exposure of parenteral products, cosmetics, medical devices, and other healthcare products during manufacturing. GMP water for pharmaceutical use, distilled water cosmetic grade, bulk water supply requirements, sterile water for injection, and types of bulk water used, as well as information on what is hemodialysis, will all be covered in this article.
What types of bulk water are used to create cosmetic and pharmaceutical products?
Purified water is created in large volumes and distributed through sterile piping systems. The following waters are bulk purified waters (GMP water) for medical products and cosmetics.
Purified Water
Purified water is used as a non-parenteral product excipient and for cleaning components and equipment that process non-parenteral products. Purified drinking water has microbial limits that must not be greater than 100 colony-forming units per milliliter (cfu/mL) to prevent endotoxin contamination. Further, purified water must meet ionic and organic chemical purity requirements. Purified water begins as drinking water quality before additional processing to contain microbial and chemical purity requirements.
Water For Injection (WFI)
GMP water for injection can be used as a parenteral product excipient and in cleaning parenteral product processing equipment. The microbial limits for water for injection are stricter than for purified water, as WFI must not have more than 10 cfu per 100 mL. WFI must also meet certain chemical limits. As with purified water, WFI begins as drinking water quality before additional processing to contain microbial and chemical purity requirements.
Water For Hemodialysis
Water for hemodialysis, as the name implies, is used for hemodialysis. Specifically, water for hemodialysis is used to dilute hemodialysis concentrate solutions. Often GMP water for hemodialysis is created and used on site. Water used for hemodialysis contains no antimicrobial agents and must meet chemical and endotoxin limits.
This water is not intended for injection. As with WFI, water for hemodialysis begins as drinking water quality before additional processing to contain microbial and chemical purity requirements.
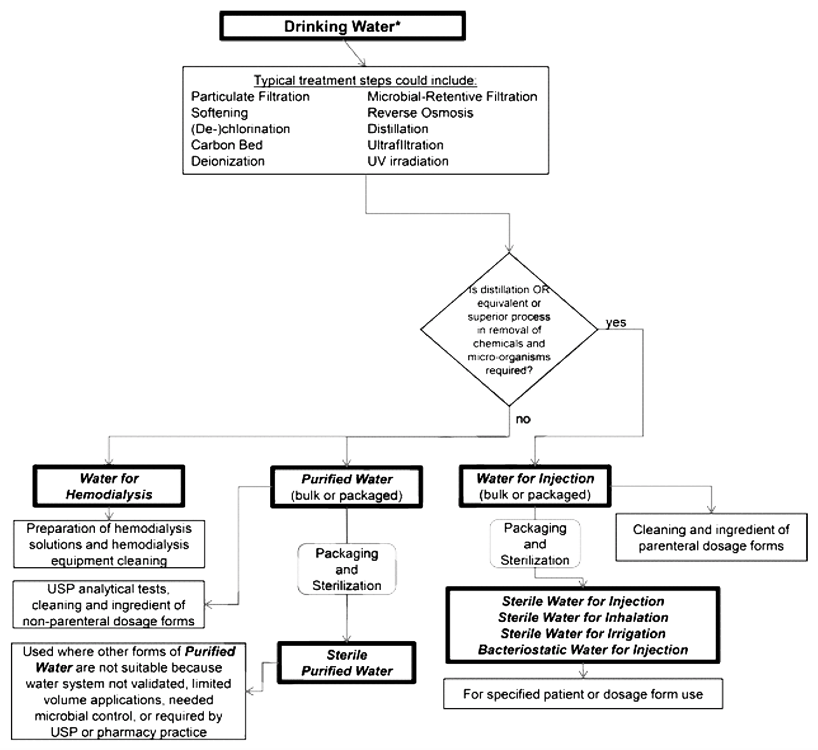
Packaged GMP waters often come from sterile bulk water sources. These packaged waters are used instead of sterile bulk water from the piping systems. Though sterile bulk and packaged water may initially come from the same piping source, the acceptance criteria for the chemical purity of packaged sterile water differs from bulk sterile water. Acceptance criteria differ because the materials and elastomeric closures used for sterile packaged water create impurities that increase in abundance over the packaged water’s shelf life. Thus, the chemical purity at the time of use must be considered when using packaged sterile water in cosmetic and pharmaceutical manufacturing. Sterile packaged waters have specific intended uses and cover a variety of packaging types, volumes, and configurations.
Sterile Purified Water
Sterile purified water is packaged purified water. Sterile purified water is used to prepare non-parenteral products or for analytical tests requiring small amounts of purified water. Sterile purified water is frequently used when a microbiological quality superior to bulk purified water is needed.
Sterile Water For Injection
Sterile water for injection is packaged and reserialized WFI. Water for injection is used as a parenteral product diluent and for prescription compounding. If access to bulk WFI or purified water is not available or practical, packaged WFI may be used. Sterile WFI is packaged in single-dose containers of one liter or less.

Bacteriostatic Water For Injection
Bacteriostatic water for injection is packaged and reserialized WFI. Bacteriostatic water contains at least one antimicrobial preservative. Multi-dose and other parenteral products utilize bacteriostatic water to reconstitute or dilute formulations. Bacteriostatic water is packaged in both single- or multiple-dose containers less than 30 mL.
Sterile Water For Irrigation
Sterile water for irrigation is WFI packaged in single-dose containers that allow rapid contents delivery. Sterile water for irrigation does not need to meet the USP 788 particulate matter requirements for injections. Sterile water applications that do not require particulate matter limits can use sterile water for irrigation to substitute purified water or water for injection.
Sterile Water For Inhalation
Sterile water for inhalation is packaged WFI used in inhalators or inhalation solutions. Sterile water for inhalation has lower endotoxin requirements than sterile water for injection. Thus, sterile water for inhalation should not be used for parenteral products.
Summary
Overall, microbes in the water can easily contaminate sterile products during manufacturing. Thus, specialized sterile water is needed to manufacture and prepare cosmetic and pharmaceutical products. The top five types of packaged sterile water used in cosmetic and pharmaceutical products are 1) Sterile Purified Water, 2) Sterile Water For Injection, 3) Bacteriostatic Water For Injection, 4) Sterile Water For Irrigation, and 5) Sterile Water For Inhalation. Water for injection and purified water are the bulk waters used to fill the top five types of packaged sterile water. All in all, ensure you choose a contract manufacturing organization that can support you with appropriate environmental monitoring, microbiology testing, and filling for your sterile product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1231> Water For Pharmaceutical Purposes. Rockville, MD, USA. 2021. (USPC <1231>).
Sharing this in your social netwroks

