What is the microbiology of air in regulatory manufacturing environments?

Why is knowing the microbiology of air important for medical product, cosmetic, or dietary supplement manufacture?
Microorganisms attached to particulates (skin particles or airborne raw material flakes) or aerosols can travel distances, survive, and further colonize remote environments. Microbes living on airborne materials can drift and contaminant manufacturing surfaces, additives, and equipment causing a regulatory hazard. Thus, it is crucial to be aware of potential microbial contaminants in the air to monitor and control them for product manufacturing. Microbiology air samplers are traditionally used in GMP manufacturing environments to collect air samples. Microbiology air quality labs or contract testing facilities then assess the microbiology of the air samples.
What is the microbiology of air in a regulatory environment?
The air in GMP manufacturing environments is dry and cool to prevent microbial growth. Thus, the airborne microbes in a regulatory environment come primarily from the airborne microbiome of the raw ingredients used for manufacture and the facility technicians. Microbiology air samplers are set-up in high-risk areas and used to capture any airborne microbes and prevent large-scale contamination. Microbiology air quality labs and contract testing companies are often used to determine both microbial quantity and type, especially if GMP manufacturing environments experience an unexpected contamination issue. Microbiology air samplers in the simplest form are petri dishes left out to collect migrant microbes. However, newer microbiology air samplers suck air up into a system and catches any airborne microbes on petri dishes or filters within the device. All air samples must be collected aseptically and delivered in sterile containers to microbiology air quality labs for assessment.
What is the microbiology of human skin?
A diverse array of bacteria, viruses, fungi, and archaea live on human skin. Typically, an adult person houses one thousand species of bacteria on the two square meters of their skin. The skin’s primary function is to protect our bodies from microbial infection or poisonous toxins. The skin’s secondary role is to interface with the outside environment, which is why it is colonized by a diverse collection of microorganisms, including bacteria, fungi, and viruses, see Figure 1. Microorganisms populate the surface of the skin, hair follicles, and sweat glands. On the skin’s surface, rod-shaped and round bacteria like Proteobacteria and Staphylococcus spp. form colonies along with fungi such as Malassezia spp. In comparison, virus particles live in bacterial cells.
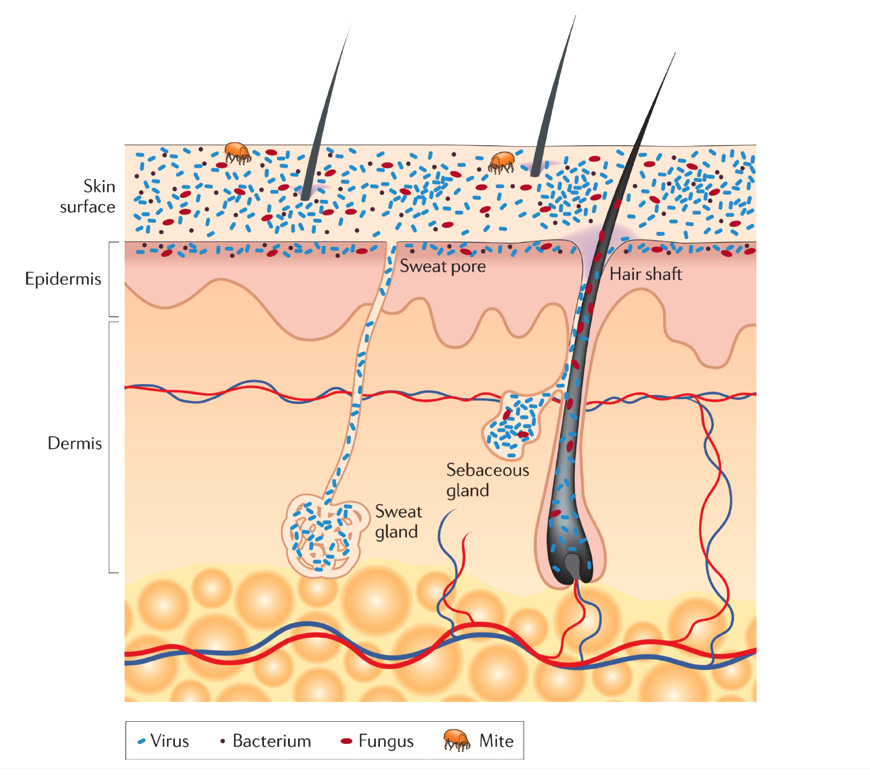
In general, skin microbiota is predominately composed of Propionibacterium spp. and Staphylococcus spp. Gram-positive bacteria. Historically, staphylococcus epidermidis is a primary bacterial colonizer of the skin along with Micrococcus. Gram-negative bacteria, except for some Acinetobacter supp. do not typically inhabit human skin. However, the skin’s microbiota varies from person to person such that different individuals only share 13% of the same bacteria on their hands. Bacteria colonization varies based on the area of the body assessed as well as from person to person. For example, the bacteria that populate the back are predominantly Propionibacterium spp. However, the elbow and the bottom of the feet are primarily composed of Betaproteobacteria and Staphylococcus spp., respectively.
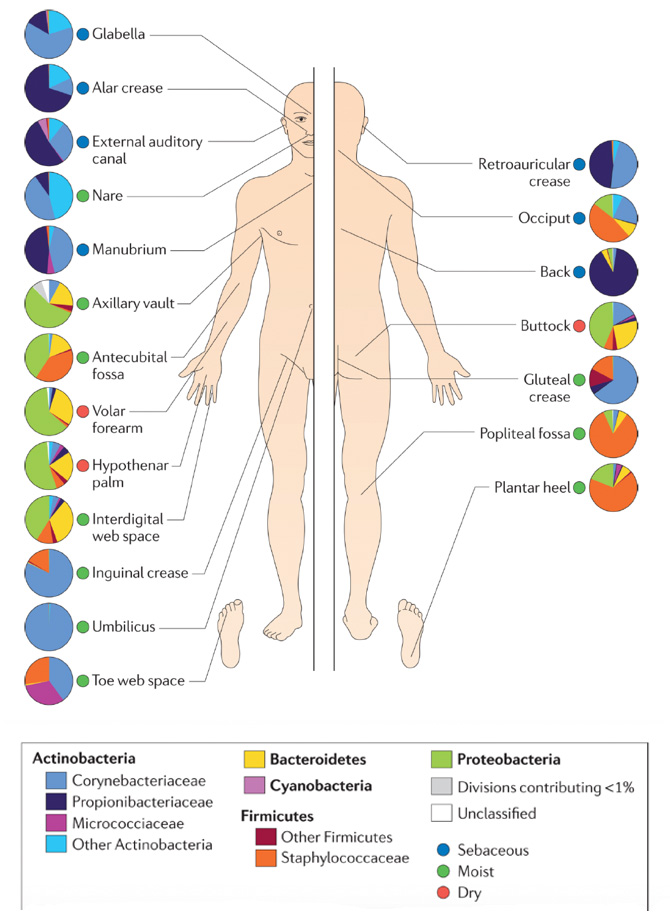
Figure 2 below depicts the distribution of bacteria at various human skin sites. The family-level classification of bacteria that colonize different body areas is shown with the organism’s phyla bolded. Locations of the skin in Figure 2 are also defined as sebaceous (oily), moist, or dry areas.
Most of the fungal microorganisms identified on the healthy skin are Malassezia spp. In one study, Malassezia spp. was measured to be fifty to eighty percent of the total fungal population of the skin. Interestingly, Candida spp., a yeast responsible for causing severe illness in hospitalized patients, rarely colonizes the human skin.
Summary
Overall, microorganisms attached to particulates (skin particles or airborne raw material flakes) or aerosols can travel distances, survive, and further colonize remote environments. Microbes living on airborne materials can drift and contaminant manufacturing surfaces, additives, and equipment causing a regulatory hazard. Thus, it is crucial to be aware of potential microbial contaminants in the air to monitor and control them for product manufacturing. The air in regulatory environments is dry and cool to prevent microbial growth. Thus, the airborne microbes in a regulatory environment come primarily from the airborne microbiome of the raw ingredients used for manufacture and the facility technicians. Common fungi and bacteria that populate the human skin are Malassezia spp., Propionibacterium supp. and Staphylococcus spp. Generally, gram-negative bacteria do not populate human skin and are not a common airborne contaminant. When it comes to dry ingredients, gram-positive spore-forming bacteria and molds are the most common microbial contaminants. Whereas damp or freshly harvested materials often house gram-negative bacteria and molds. All in all, ensure you choose a contract testing organization that can support you with appropriate environmental monitoring and microbiology testing for your unique medical device or product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Elizabeth A. Grice and Julia A. Segre. The skin microbiome. Nat Rev Microbiol. 9(4): 244–253. 2011.
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Sharing this in your social netwroks

