Excipient Cytotoxicity Testing For Cosmetic Or Parenteral Products
What is cytotoxicity testing?
Cytotoxicity refers to molecules and compounds that are poisonous to living cells. Cytotoxins are often chemical but can also be from natural or biological sources. Cytotoxicity testing evaluates the biological reactivity of mammalian cells and tissues to contact with elastomeric plastics, excipients, and other materials that will come in direct or indirect patient contact during medical product use. Cytotoxicity is significant as it evaluates the biological effects of a sample’s leachable chemicals. The types of cytotoxicity testing to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within. In-vitro USP 87 methods of cytotoxicity testing include direct contact, agar diffusion, and elution testing. In-vivo USP 88 methods of cytotoxicity testing include intracutaneous injection, systemic, and implantation testing. Most medical devices and products will only require in-vitro cytotoxicity testing.
What are excipients?
Medical-grade excipients are agents designed to support a drug or cosmetic preparation functionally and have limited (or no) pharmacological activity. Diluents or stabilizing agents are examples of excipients and their functions. Medical-grade excipients fall under two categories: excipients that have not been previously permitted for use and excipients that have been previously used in pharmaceutical preparations. This article covers what will be needed to prove the safety of a new excipient intended for use in parenteral products.
Where can you find toxicity data for new medical-grade excipients?
Excipient toxicity data can come from their use in human foods or products used by animals. However, excipients used for inhalation, injection, and other sensitive areas of the body will require toxicity data beyond oral consumption or animal use. Toxicological and chemical databases can be used to find information on most chemicals and their metabolites.
What are the toxicity requirements for new medical-grade excipients?
Toxicity data requirements are dependent upon the intended use, frequency of use, duration, and dosage of the excipient. For toxicity evaluations, information on the excipient’s physical and chemical properties, its manufacturing process, impurities limits, pharmacological activity, genotoxicity, and exposure conditions is needed. Areas of interest for toxicity are standard absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME/PK) studies. In cases where the excipient is in a short half-life product without excipient build-up risk, background information and baseline toxicity information alone may support using the new excipient. Table 1 below summarizes excipient toxicity testing requirements based on a product’s route of exposure. Required tests are denoted with (R), and conditional tests are denoted with (C).
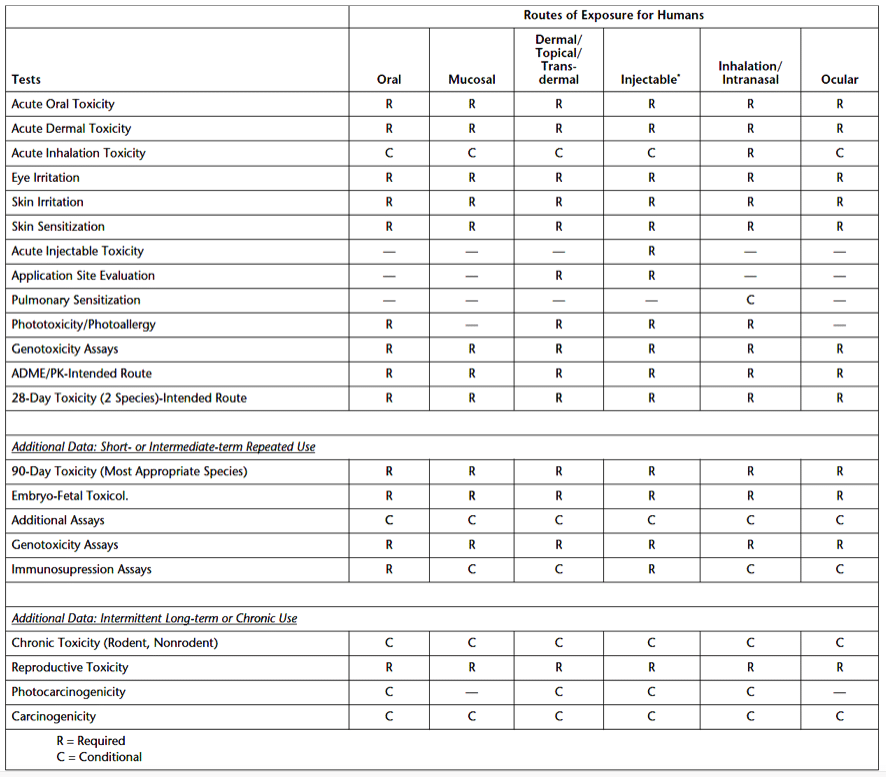
What are the exposure categories for parenteral product excipients?
#1: Acute Exposure
Acute exposure falls within a 24-hour exposure period. Doses of excipients may be single, multiple, or continuous during the 24-hour period.
#2: Subacute Exposure
Subacute exposure involves repeated exposure to a test agent for up to twenty-nine days. Daily doses may be single, multiple, or continuous in 24-hours.
#3: Sub-chronic Exposure
Sub-chronic exposure is repeated exposure to a test agent for thirty days. For animals, thirty days is considered equivalent to ten percent of the lifespan of the test species. Daily doses may be single, multiple, or continuous during a 24-hour period like other exposure categories.
#4: Chronic Exposure
Chronic exposure is considered repeated exposure to a test agent for more than ten percent of the lifespan of a test species. These would be long-term use products. As with sub-chronic exposure, daily doses may be single, multiple, or continuous during a 24-hour period.

What are the required baseline toxicity data needed for parenteral product excipients?
The following toxicity data are needed for new excipients:
- Acute toxicity testing by intended dose routes such as skin sensitization, approximate lethal dose method, and limit tests
- Acute toxicity studies such as skin irritation and oral toxicity by limit testing.
- ADME/PK: single or multiple doses
- Genotoxicity testing such as Ames testing, in-vitro chromosome aberration test, and mammalian cell mutation assay.
- Repeated dosing studies (28-day) in two species by appropriate routes (one rodent, one mammalian nonrodent). An evaluation of the injection site may be needed for these studies.
For repeated dosing studies, the comparison of toxicity and ADME/PK data between oral and intended routes is critical for other toxicity testing requirements (e.g., reproductive toxicity testing might be assessed via an oral route rather than the intended route of administration).
What additional toxicity data are needed for excipients used in non-oral or mucosal therapies?
Additional Toxicity Data For Dermal, Topical, & Transdermal Exposure
- Assess effects of acute transdermal exposure, including a dermal sensitization study for repeat applications.
- Assess the effects of repeated transdermal exposures, including a photoallergy-phototoxicity study and studies in two species (one rodent, one mammalian nonrodent).
- Assess the effects of sub-chronic exposure and reproductive toxicity.
- Photocarcinogenicity studies may be required for prolonged skin exposure, for skin drug concentrations that exceed plasma drug concentrations, or for products with photo-activity.
Additional Toxicity Data For Injectable Dosage Forms
- Show data on the effects of acute exposure, including an evaluation of injection site irritation in rabbits or dogs and an evaluation of the injection administration rate.
- Define compatibility of the injectable formulation with blood (if exposed to the circulatory system).
- Define the pH and tonicity of the injectable formulation.
Additional Toxicity Data For Inhalation Or Intranasal Exposure
- Assess acute inhalation toxicity that includes a limit test that uses the highest achievable concentration in a 4-hour exposure to vapor, aerosol, or solid particulate and a pulmonary sensitization test.
- Perform a single and repeated dose ADME/PK by inhalation or intranasal/oral routes.
- Perform a repeated dose inhalation study (28-day) in two mammalian species using vapor or particulates and compare the results of this study to similar oral toxicity data.
Additional Toxicity Data For Ophthalmic Exposure
- Define the pH and osmolarity for topical ocular formulations.
- Assess the effects of acute exposure, including cytotoxicity tests (e.g., agar overlay)
- Assess the effects of repeated exposures, including studies in two species (one rodent, one mammalian nonrodent) and studies on allergenicity potential. Examinations of anterior and posterior segments of the eye are required.
Summary
Overall, pharmaceutical excipients are agents designed to functionally support a drug or cosmetic preparation but to have limited or no pharmacological activity. Diluents or stabilizing agents are examples of excipients and their functions. Medical-grade excipients fall under two categories: excipients that have not been previously permitted for use and excipients that have been approved for other preparations. This article covers what will be needed to prove the safety of a new excipient intended for use in cosmetic or parenteral products. Toxicity data requirements are dependent upon the intended use, frequency of use, duration, and dosage of the excipient. Areas of interest for toxicity are standard absorption, distribution, metabolism, excretion, and pharmacokinetics (ADME/PK) studies.
In cases where the excipient is in a short half-life product without excipient build-up risk, background information and baseline toxicity information alone may support the use of the new excipient. Excipients used for inhalation, injection, and other sensitive areas of the body will require toxicity data beyond oral consumption or animal use. Ensure you choose a contract manufacturing organization that can support you with appropriate toxicity testing for your parenteral product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Cytotoxicity Testing, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1074> Excipient Biological Safety Evaluation Guidelines. Rockville, MD, USA. 2021. (USPC <1074>).
Sharing this in your social netwroks

