How To Perform Cleaning Validations For Reusable Devices
What are cleaning validations?
Cleaning validations are protocols that ensure items, equipment, or areas are consistently cleaned to certain acceptance criteria. For reusable devices, cleaning validations prepare devices to undergo sterilization and ensure that tissues, proteins, lipids, bacteria, and cellular debris are removed from used devices to acceptable levels. Cleaning validations have internationally accepted standards that must be met. These cleaning standards can be verified using test soils that simulate contaminants that the reusable devices would collect during use. For example, cleaning validations specify how contaminants like blood, tissues, and feces are removed from reusable medical devices during reprocessing.
What is the importance of cleaning validations for reusable devices?
Medical devices are extremely diverse in their shape, size, and use. Reusable medical devices require cleaning, disinfection, and sterilization before they can be used again. Each of these processes (cleaning, disinfection, and sterilization) must be validated to ensure patient safety and prevent hospital-acquired infections due to inadequate cleaning (and subsequent inadequate sterilization) of reusable devices.
What are the keys to a successful cleaning validation?
Cleaning validations are used to simulate “worst-case” contaminant conditions and verify that certain cleanliness levels can be met consistently following a set cleaning protocol. Generally, the following six steps are taken to set up a successful cleaning validation. At least 10 devices are tested during cleaning validations.
#1: Define the reusable device and device family (if applicable)
Product families play a role in defining cleaning validations. Medical devices with common attributes and features can be grouped into a product family. Product families only require a single validated cleaning process and acceptance criteria for the entire family of products. The medical device within a product family used for cleaning validation testing should be the most difficult device to clean. Selection criteria for this “worst-case scenario” medical device (often referred to as the master product) must be documented so that the FDA and other governing bodies can verify the selection rationale. An example of a “worst-case scenario” medical device within a product family would be the longest endoscope within a product family, where all features are the same except for the length. After a master product is verified, additional members of the product family can be validated by equivalency.
Outside of product family considerations, other factors that define reusable devices for cleaning validations are:
- Is the device made of multiple material types?
- What materials is the device made of?
- How do the device materials affect adhesion, chemical interaction, porosity, etc.?
- Is the device design complex?
- Does the device have mated surfaces, hinges, angles, lumens, channels, crevices, shielded areas, serrations, threaded areas, or rough surfaces?
- Can the device be disassembled for cleaning?
- Can the lumens and channels easily undergo thorough cleaning?
- Where are contaminant residuals likely to settle (e.g., within crevices)?
- What parts of the device will be contaminated during device use?
- Are there areas where cleaning solutions and tools cannot easily reach (e.g., angled lumens)?
- How will improper cleaning of hard-to-reach areas affect device functionality?
#2: Identify clinical contaminants
In order to assess clinical contaminants, data on bodily areas the device will touch, parts of the device that will be in contact with contaminated body fluids, the types of body tissue and fluids contacting the device during use, and any chemical contaminants during device use and reprocessing are accounted for. Chemical contaminants might be lubricants, drying aids, detergents, etc.
Common body tissues and fluids that contact medical devices are: skin, liver, brain, blood, mucus, bone, fat, cartilage, cerebrospinal fluid, and fecal matter.
#3: Choose an appropriate test soil
No single test soil is appropriate for all medical devices due to their unique methods of use. The ratios, viscosities, and densities of the applicable body tissues and fluids in contact with the device must be assessed to determine an appropriate test soil. The chemical composition of worst-case scenario soil (e.g., types of proteins, bacteria, virus, volumes, and ratios) must be considered. Literature reviews are often performed to support creating representative physical and chemical compositions for the cleaning validation test soil. In cases (such as endoscopes) where bacteria may be encountered, bacterial soil challenges are often 1×104 colony-forming units per device.
#4: Select appropriate methods for contaminant removal
TIR 30 is the penultimate cleaning document for the medical device industry and contains charts with test methods and acceptance criteria for cleaning reusable medical devices. Additional specifics on test methods can be found in TIR 30. Additionally, it is important to determine how long the device will sit with dried soil between patient use and cleaning when determining contaminant removal processes and presoak, scrubbing, or rinsing times.
The following are important considerations for contaminant removal:
- Challenge material—soil and bodily fluid choices
- Cleaning fluid selection
- Cleaner type—enzymatic category (lipase, proteinase)
- Presoak/soak contact time and water temperatures
- Water quality (pH and hardness)
- Manual cleaning brush criteria
- Precleaning rinse (if needed, time, temperature, etc.)
- Post-cleaning rinse (if needed, time, temperature, etc.)
- Drying time and temperature
- Pass/fail criteria—endpoint analysis
Manual cleaning elements are:
- Hydration—adding water to the device and causing the extraneous material to absorb water molecules
- Friction— providing mechanical energy to remove extraneous material
- Digestion—using chemicals or proteinase/lipase enzymatic cleaners to remove material by breaking down large molecules into smaller, water-soluble ones
- Solubilization—using a chemical process to alter extraneous materials’ surface characteristics and increase their affinity for water
- Fluidics—using water to move extraneous material
Cleaning times for selected cleaning methods should be the worst-case during the validation. For example, soil drying times should be the maximum amount to allow the soil to adhere or absorb into the device as much as possible. On the other hand, rinse times and manual scrubbing times should be performed for the minimum time in the validation protocol.
#5: Select appropriate methods to test for residual soil contaminants
Soils can be evaluated through visual inspection and lab-based test methods. If performing visual soil inspection, determine if the visual inspection should be with magnification, without magnification, or with a borescope (for internal components such as device lumens). Further, manufacturers must decide if biomarkers or other contaminants will be metrically assessed to ensure device cleanliness. If metrics are needed, available test methods and their detection levels will need to be assessed. Test methods must have an adequate detection level to measure each specific soil marker acceptance criteria to the required precision. In some cases, detergent chemical residues on devices are assessed following the completion of the cleaning validation rinsing process. With frequently used devices, cleaning chemicals and scrubbing tools may need to be assessed to assure that they will not affect device function after multiple rounds of use. National standards and scientific literature can be used to determine a justified “standard” for test methods, soil markers, and chemical residue expectations.
As a part of soil test method selection, a method must be created for contaminating the device with the worst-case scenario test soil. Test method results must be expressed in the same units as the set acceptance criteria. Further, extraction volumes for soil assessment must be large enough for the test method selected. Otherwise, post-cleaning soil samples will fall below the level of detection for the test method. Generally, the level of detection for tests is set at three times the standard deviation of the negative control.
#6: Set scientifically justified acceptance criteria
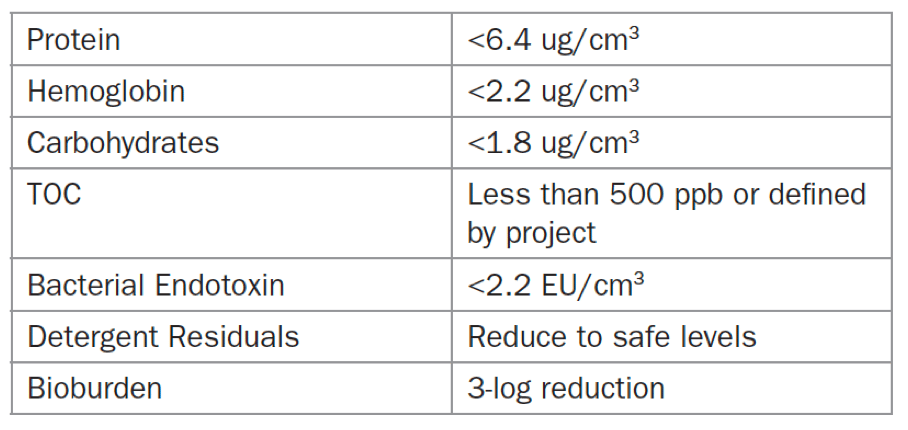
First, set checkpoints for examining devices for visual cleanliness. Then, document information on toxicology, biocompatibility, and other device elements that could be hazardous to patient safety. Next, show that the soil marker levels are acceptable based on TIR/ISO requirements and scientific literature. Prove that any device lumens and channels can be cleaned effectively. Some methods for cleaning these elements are flushing or using a borescope. Examples of acceptance criteria for microbes (bioburden), proteins, and other soil components are given in Table 1 below.
What are simulated accumulation studies?
Simulated accumulation studies are often part of a cleaning validation study and are required for most devices. Simulated accumulation studies test the device reuse life. In other words, simulated accumulation studies determine how many times the device can be reprocessed and reused. The standard for simulated accumulation is repeating soiling and cleaning validation reprocessing on the same device(s) ten times. Simulated accumulation studies for cleaning validations can be linked with sterilization to test the device reuse cycle from patient use through re-sterilization.
Summary
Overall, reusable medical devices require cleaning, disinfection, and sterilization before being used again. Each of these processes (cleaning, disinfection, and sterilization) must be validated to ensure patient safety and prevent hospital-acquired infections due to inadequate cleaning (and thus sterilization) of reusable devices. Cleaning validations specify how blood, tissues, and feces are removed from reusable medical devices during reprocessing.
Cleaning validations are used to simulate “worst-case” contaminant conditions and verify that certain cleanliness levels can be met consistently following a set cleaning protocol. Generally, cleaning validations follow the following steps: 1) Define the reusable device and device family; 2) Identify clinical contaminants; 3) Choose an appropriate test soil; 4) Select appropriate methods for contaminant removal; 5) Select appropriate methods to test for residual soil contaminants; 6) Set scientifically justified acceptance criteria. MycoScience specializes in quickly creating effective cleaning validations for their client’s reusable components and devices. Ensure you choose a contract testing organization that can support you with your unique cleaning validation needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling for parenteral products. MycoScience also offers testing services, including Preservative Efficacy Testing/Suitability Testing, Bioburden Testing, Microbial Aerosol Challenge Testing, Cytotoxicity Testing, Cleaning Validations, Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Association for the Advancement of Medical Instrumentation Technical Information Report. A compendium of processes, materials, test methods, and acceptance criteria for cleaning reusable medical devices. Arlington, VA, USA. AAMI; 2011. (AAMI TIR30-2011).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Steven G. Richter. Cleaning Validations of Medical Products. Chapter 10: The Medical Device Validation Handbook 2nd Edition. RAPS.
Sharing this in your social netwroks

