Qualitative Equivalency Methods For Microbiological Testing
What is method equivalency testing?
FDA equivalency testing (method equivalency testing) determines whether an alternative test method is equivalent to a USP general test method (i.e., USP 51, USP 61, USP 62, USP 63, and USP 71). An alternative method need only demonstrate equivalency to a USP test with one product to prove qualitative microbiology equivalency. However, method suitability will need to be determined for each new product evaluated with the equivalent alternative microbiological method.
Alternative microbial method validation should involve:
- Equivalence demonstration
- Analytical method and equipment qualification
How do you demonstrate FDA equivalency?
Alternate methods must provide proven advantages in accuracy, sensitivity, precision, selectivity, adaptability to automation, or computerized data reduction before they can be used instead of compendial methods. In other words, alternative methods must produce statistically equivalent or better (non-inferior) results than the compendial method.
How do you establish alternative analytical FDA equivalency?
- Acceptable procedures (i.e., meeting a minimum performance or acceptance requirement without a need to demonstrate equivalence to the compendial method)
- Performance equivalence to the compendial method
- Results equivalence to the compendial method
- Decision equivalence to the compendial method
How do you establish alternative qualitative microbiology equivalency?
Results using methods in USP 62, USP 63, and USP 71 are pass/fail qualitative tests that indicate the presence or absence of microorganisms in the tested sample. Equivalency (method acceptability) can be proven for qualitative tests using approach 1 and approach 2, detailed below. Approach 1 below is based on demonstrating decision equivalence. Approach 2 below is an alternative that converts the qualitative results to quantitative ones using the most probable number (MPN) procedure.
Approach 1: Use of Presence & Absence Qualitative Microbiology Results
The proportion of samples producing a signal for the new procedure (PN) is not more than some amount (Δ > 0) less than the proportion for the current, compendial procedure (PC) to prove non-inferiority for approach 1.
Result = PN − PC ≥ −Δ
Where:
PN= new procedure
PC= compendial procedure
Δ= the non-inferiority margin
Typically, the non-inferiority margin is set at Δ = 0.20. Thus, for a one-sided 90% confidence interval for PN – PC, non-inferiority is concluded if the lower confidence limit exceeds −0.20. Three evaluations are made for each representative microorganism tested with alternative and compendial methods with different microorganism concentrations. Each evaluation uses a different microbial concentration. The first concentration is around 1 colony-forming unit (cfu), which simulates the point where no microbial growth is likely to be detected. The second evaluation is between 100-200 cfu, where microbial growth is expected in 75% or more samples. The last evaluation is made at a cfu of 10-50, where 50-75% of samples would be expected to grow colonies. For non-inferiority, 75 samples are tested at these three microorganism concentrations. If the new method is less sensitive than the approved USP method (compendial method), testing with 100 samples is performed to see if non-inferiority criteria can be met.

Approach #2: Compare Qualitative Microbiology Most Probable Number Results
A most probable number (MPN) comparative study creates MPN values for each sample to prove FDA equivalency. Using the same samples for two procedures yields the best results but is not required.
For MPN comparison, the non-inferiority hypothesis is:
μA − μC ≥ log(R) or antilog (μA − μC) ≥ R
Where:
μA = the means in the log scale for the alternative procedure
μC = the means in the log scale for the and compendial procedures
For MPN of independent data values, conclude non-inferiority if antilog(L low) ≥ R in the equation below for independent samples.
Use the equation below to calculate L low for independent data values:
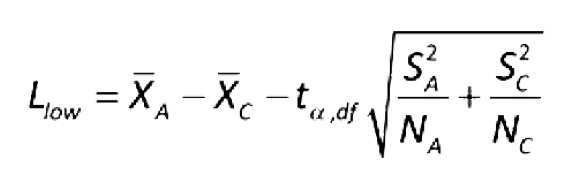
Where:
tα, df = the upper α percentage point of the t distribution with df degrees of freedom (linear interpolation may be needed)
NA = number of samples using an alternative method
NC = number of samples using compendial method
XA = sample mean of the log values for an alternative method
XC = sample mean of the log values for compendial method
S2A = sample variance of the log values for an alternative method
S2C = sample variance of the log values for compendial method
Where:
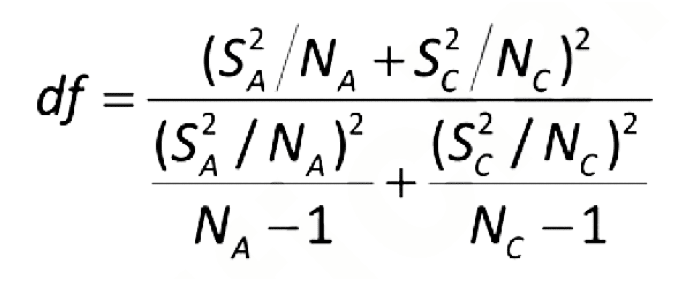
For MPN of paired data values, use the equation below. Non-inferiority is met if antilog(L low) ≥ R.
Use the equation below to calculate L low for paired data values:
L low = x − tα,N −1 S/√ N
Where:
tα, N −1 = the upper α percentage point of the t distribution with N − 1 degrees of freedom
Equivalence Demonstration For Quantitative Microbiological Procedures
When alternative method signals differ from the colony-forming unit (cfu) of the compendial microbiological procedures, two verification methods may be used.
These verifications for quantitative methods are:
- Results from the alternative quantitative procedure have acceptable repeatability.
- Results from the alternative quantitative procedure are highly correlated (95% confidence) with cfu.
Summary
All in all, a method equivalency test determines if a validated alternative microbiological method is equivalent to a USP general test method (e.g., USP 51, USP 61, USP 62, USP 63, and USP 71). Method equivalency for an analytical method can be proven through four means: acceptable procedures, performance equivalence, results equivalence, or decision equivalence to the compendial method. There are two ways to calculate method equivalency: approach 1, which compares alternative and compendial method signals, and approach 2, which uses the most probable number method. MycoScience can perform FDA-approved equivalence and suitability testing. Method equivalence is important if your device requires a unique microbiological testing evaluation or if compendial methods are being modified for time/cost savings. Ensure you choose a contract testing organization that can support you with appropriate suitability testing for the regulatory needs of your product.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling for parenteral products. MycoScience also offers testing services, including Preservative Efficacy Testing/Suitability Testing, Bioburden Testing, Microbial Aerosol Challenge Testing, Cytotoxicity Testing, Cleaning Validations, Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1223> Validation Of Alternative Microbiological Methods. Rockville, MD, USA. 2022. (USPC <1223>).
Sharing this in your social netwroks

