How to perform microbial enumeration testing for dietary supplements
What are dietary supplements?
Dietary supplements are growing in popularity as bulk production of foods leads to nutritional deficiencies. Dietary supplements are being manufactured to supplement elements of our diets to combat these nutritional deficiencies. Examples of supplements include one or a combination of botanicals, amino acids, minerals, vitamins, and enzymes. Supplements come in multiple forms, including oral tablets, powders, encapsulated pills, and liquids. This article covers information on microbial enumeration tests (including enumeration testing for dietary supplements) and microbial enumeration methods, such as the multiple-tube testing method. However, specific FDA dietary supplement microbial limits are not covered in this article.
Why is microbiological testing and monitoring necessary for dietary supplements?
Microbiological testing, monitoring, and microbial enumeration methods are critical to keeping harmful bacteria, yeast, and molds from supplement products. Products that don’t meet appropriate microbial limits are dangerous and risk causing patient illness upon use. Microbial contaminants enter the manufacturing process in a variety of ways. The first and most common way is through the raw materials, pharmaceutical ingredients, and active ingredients used in nutritional and dietary supplement manufacture. Indeed, supplement components received from suppliers are not sterile. In particular, botanicals may be microbiologically contaminated with human or animal feces and molds at any point during growth, harvesting, processing, packing, or distribution. Thus, the level of microbiological contamination (bioburden) for a supplement’s raw ingredients must be monitored during manufacture. This monitoring prevents patient illness upon dietary supplement use. The second way microbial contaminants enter supplement products is during the manufacturing process. Microbial contaminants can enter manufactured products through facility environments (human operators & technicians), manufacturing equipment, and water use. Due to the contamination risks during manufacture, reliable dietary supplements are processed under Good Manufacturing Practice (GMP) protocols. These GMP protocols control raw material bioburden, facility environments, equipment cleaning, water sources, and manufacturing process to minimize microbial content and contamination during product manufacture.
How frequently are microbiological tests performed?
In terms of enumeration testing for dietary supplements frequency, microbe sampling and and microbial enumeration methods vary based on the type of supplement and its route of administration. Examples of other products that need less routine microbial testing include highly refined botanical extracts and products with low water activity. In some cases, such as with dry oral dosage forms, little testing is necessary. In other cases, such as oral liquid dosage forms, periodic monitoring may be warranted.
Items to consider when designing microbial sampling and testing plans for supplements are:
- Route of administration of the supplement (oral, nasal, etc.)
- Microbial level of raw materials (dried herb vs. highly refined botanical extract)
- Product type (tablet, powder, pill, liquid, etc.)
- Method of manufacture and the potential for contamination during processing
- Growth promotion or inhibition properties of the supplement formulation (such as water content)
- The target population for the supplement
- Batch-to-batch supplement variability
How are microbiological tests performed?
Microbiological tests ( including enumeration testing for dietary supplements) quantify the total aerobic microbial count (TAMC), and total combined yeasts and molds count (TYMC) in colony-forming units (CFU) for supplements and their constituents. The microbial enumerations tests that quantify TAMC and TYMC include membrane filtration, plate, and multiple tube testing methods. When quantifying TAMC or TYMC, membrane filtration or plat methods should be used for freely soluble samples. All other samples should use the multiple-tube method. All three microbial quantification procedures are described in detail below.
Membrane filtration method
First, ensure that the sample is diluted to a concentration expected to grow 30-300 colonies. Next, pipet one milliliter (1 mL) of the diluted sample into Phosphate Buffer, Fluid Soybean–Casein Digest Medium, or Fluid Casein Digest–Soy Lecithin–Polysorbate 20 Medium. Then filter the diluted sample solution, wash the membrane with the selected sterile diluent, transfer the filtration membrane to a Petri dish with Soybean–Casein Digest–Agar Medium, and incubate the plate. This test should be completed with two filter membranes. The microbial colony count is expressed as an average of the two membranes in microorganisms per gram (g) or milliliter (mL) of the specimen. Suppose microbial colonies aren’t recovered from the dishes at a 1:10 dilution of the supplement. In that case, the results are recorded as less than ten microorganisms per g (or per mL) of the sample.
Plate method
First, ensure that the sample is diluted to a concentration expected to grow 30-300 colonies. Next, a milliliter of the prepared sample is added to two Petri dishes with 15 to 20 mL of Soybean–Casein Digest Agar. Petri dishes are then incubated, and the number of colonies on the plates is counted. The colony count is expressed as an average number between the two plates in microorganisms per g or per mL of specimen. Suppose microbial colonies aren’t recovered from the dishes at a 1:10 dilution of the sample. In that case, the results are recorded as less than ten microorganisms per g (or per mL) of the sample.
Multiple-tube method
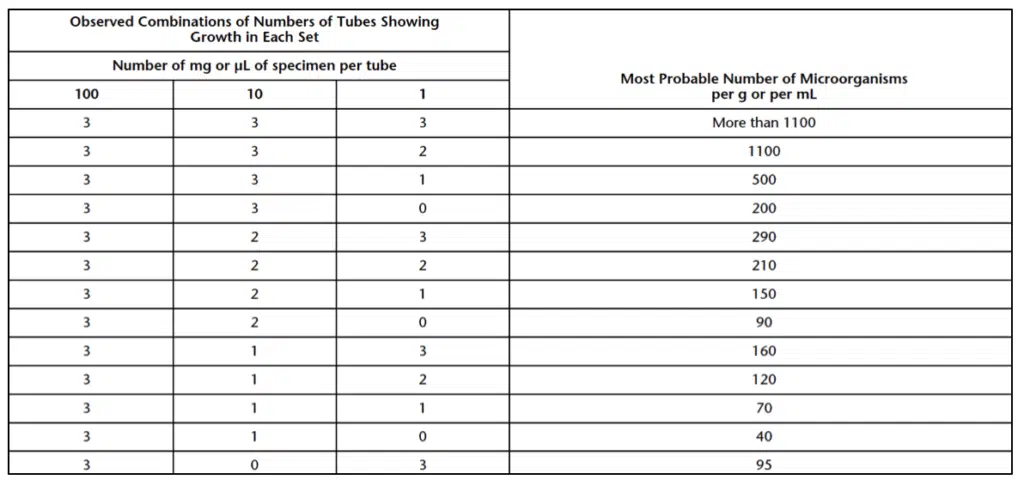
For the multiple-tube method, sterile Soybean Casein Digest Medium (9mL) is added to fourteen test tubes of similar size. The multiple-tube testing methods uses twelve of the fourteen tubes placed in four sets of three tubes. One set is media controls, the second set is a 100-dilution of the sample, the third set is a 10-dilution of the sample, and the fourth set is a 1-dilution of the sample. Pipet 1 mL of the sample into each of the three tubes of set two (100-dilution) and a fourth tube (tube A) and mix. Then, use tube A’s dilution to pipet 1 mL from tube A into the third set of three tubes (10-dilution) and a fourth tube (tube B) and mix. Finally, used the dilution from tube B to pipet 1mL from tube B into the fourth set of three tubes (1-dilution) and mixed. The remaining solutions in tubes A and B can then be discarded. At the end of the dilution series, three tubes with a 100 milligram (mg) or 100 microliter (μL), three tubes with 10 mg or 10 μL, and three tubes with 100 mg or 100 μL dilution of the sample should remain. These nine tubes are then incubated along with the three media controls. After incubation, the tubes are examined for growth using Table 1, indicating the most probable number of microorganisms per g or mL. All control tubes for the multiple-tube testing method should remain clear following incubation, or the multiple-tube testing method will need to be repeated.
Total aerobic microbial count (TAMC) vs. total combined molds and yeasts count (TYMC)
Soybean– Casein Digest Agar is used for microbial enumeration testing for total aerobic microbial counts (TAMC) and Sabouraud Dextrose Agar for total yeast and mold counts (TYMC). Ensure the appropriate agar is used for TYMC and TAMC assessment. Saubouraud Dextrose Agar can be substituted for the same amounts as Soybean– Casein Digest Agar for TYMC testing.
How do you prepare supplement samples for microbial enumeration testing?
To prepare the samples for any of the three microbial quantification methods, dissolve or suspend ten grams or ten milliliters of the sample to make one hundred milliliters of stock. Viscous supplements may need to be diluted further before they are able to be pipetted. Phosphate Buffer, Fluid Soybean–Casein Digest Medium, or Fluid Casein Digest–Soy Lecithin–Polysorbate 20 Medium can be used for sample dilutions. As the validity of microbial enumeration test results depends upon microbes being able to proliferate, sample cultures will be tested against stock microorganisms to assess their growth capabilities and test for any inhibitory (antimicrobial properties). Sample cultures with a greater than 70% bioburden recovery compared to a control medium may proceed with microbial enumeration testing. However, if bioburden recovery is lower, inactivating agents may need to be added to neutralize inhibitory substances present in the samples. Examples of inactivating agents are soy lecithin, 0.5%; and polysorbate 20, 4.0%. If inactivating agents don’t work, the samples likey have bactericidal or bacteriostatic activity. This activity can be documented and indicates the supplement is not likely to allow microbial proliferation or contamination.
Summary
Overall, dietary supplements are being manufactured to supplement elements of our human diets to combat nutritional deficiencies. Microbiological tests quantify the total aerobic microbial count (TAMC), and total combined yeasts and molds count (TYMC) in colony-forming units (CFU) for supplements and their constituents to ensure that supplements are safe for human consumption. The microbial enumerations tests that quantify TAMC and TYMC include membrane filtration, plate, and multiple tube testing methods. If samples are easily soluble, membrane filtration and plate methods are used for TAMC and TYMC assessment. Otherwise, multiple tube testing is performed. All in all, make sure you choose a contract manufacturing organization that can support you with appropriate microbiology testing for your dietary supplements.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
U.S. Food & Drug Administration. What You Need to Know About Dietary Supplements. 2017.
United States Pharmacopeial Convention. <2022> Microbial Enumeration Tests Nutritional And Dietary Supplements. Rockville, MD, USA. 2021. (USPC <2021>).
United States Pharmacopeial Convention. <2022> Microbiological Procedures For Absence Of Specified Microorganisms- Nutritional And Dietary Supplements. Rockville, MD, USA. 2021. (USPC <2022>).
United States Pharmacopeial Convention. <2023> Microbiological Attributes Of Nonsterile Nutritional And Dietary Supplements. Rockville, MD, USA. 2021. (USPC <2023>).
Sharing this in your social netwroks