How To Perform USP 51 Antimicrobial Testing
What are antimicrobial preservatives?
Antimicrobial preservatives are agents added to aqueous products to prevent microorganism growth. Antimicrobial substances are used to support shelf life and to protect liquid products from the growth of any organisms accidentally introduced to the product during or after the manufacturing process. Most antimicrobial agents are somewhat toxic to humans. Thus, antimicrobial preservatives should be used within ranges that are safe for human consumption. USP 51 governs FDA antimicrobial testing regulations for antimicrobial products such as medical devices, cosmetics, and baby wipes.
When are antimicrobial preservatives used?
Multidose therapies contain more than one therapeutic dose in a single vial. Sterile multidose parenteral products are expected to contain antimicrobials to prevent the growth of microorganisms added to the sterile product when repeatedly withdrawing individual doses. Other parenteral or nonsterile products, especially multidose oral liquids, may utilize antimicrobials to support a product’s shelf life. Due to their toxicity, antimicrobial preservatives are not a substitute for good manufacturing practices or appropriate sterilization processes. If used, antimicrobial preservatives must be declared on a product’s label.
What is antimicrobial testing, and when is antimicrobial testing needed for parenteral and non-sterile products?
USP 51 antimicrobial testing assesses how effective antimicrobial agents are at preventing microbial growth. All injectable products packaged in multidose containers must demonstrate antimicrobial effectiveness, whether the antimicrobial activity comes from inherent characteristics of the product formulation or from the addition of a known antimicrobial agent. Antimicrobial effectiveness must be demonstrated for aqueous-based, multidose topical and oral formulations. Also, ophthalmic, otic, nasal, irrigating, and dialysis fluids require antimicrobial activity.
What types of products need antimicrobial testing?
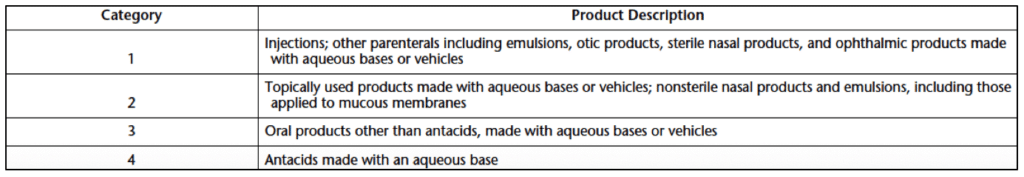
There are four categories of products that require USP 51 antimicrobial testing. Antimicrobial effectiveness criteria for each product category are based on the product’s route of administration. All four product categories are described in Table 1 below.
How is antimicrobial testing for sterile parenteral products performed?
Antimicrobial testing involves challenging a product with microbes, promoting microbial growth, and recovering the microbial concentration at various time points. Microbes used for the challenge are likely contaminants to the product during production or intended use. Antimicrobial testing is performed on products in their original, sealed containers. The first step in the testing process is preparing a microbial suspension with the challenge organism(s).
Standard USP 51 antimicrobial tests use the following microorganisms for challenging:
- Candida albicans (ATCC No. 10231)
- Aspergillus brasiliensis (ATCC No. 16404)
- Escherichia coli (ATCC No. 8739)
- Pseudomonas aeruginosa (ATCC No. 9027)
- Staphylococcus aureus (ATCC No. 6538)
Each bacterial and fungal test strain is grown separately under the conditions specified in Table 2 below.
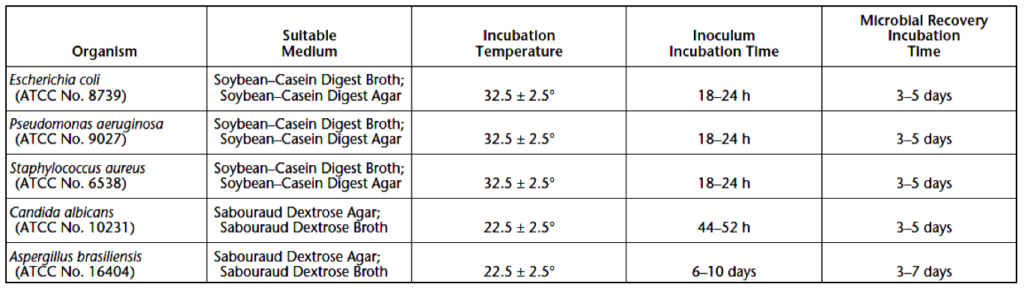
The challenge microorganisms should be in a logarithmic growth phase when used, except for A. brasiliensis spores. Microorganism cultures can be harvested using a wash with sterile saline TS or sterile saline TS with 0.05% of polysorbate 80. Each spore suspension should be aseptically treated and prepared so that no residual growth medium is contained in the suspension. The microbial suspensions used for inoculations should contain approximately 1 x 108 colony forming units (CFU) per milliliter (mL). Plate count should confirm challenge organism concentrations, even if turbidimetric procedures are initially used for microbe estimation. Media used in antimicrobial testing must be tested to verify it can support normal microbial growth. For solid media, microbial counts during growth must be at least 50% of the expected number for a standardized microbe suspension.
A product’s natural antimicrobial activity is tested by creating a solution with one milliliter of the product (by volume) and nine milliliters of saline. A small number of challenge organisms are added so that the plated product-challenge organism suspension has less than 250 CFU/plate for bacteria and yeast. Saline is inoculated and plated as a control. Microbe recovery should be at least 50% of the saline control count for products with no innate antimicrobial activity. For products with low-level antimicrobial activity, neutralizers can be added to the product to support antimicrobial effectiveness testing of added antimicrobial agents. If antimicrobial activity is naturally high, a full antimicrobial effectiveness test can be performed to determine the log unit reduction of the product’s natural antimicrobial activity.
Products are either tested in five original packaging containers or sterile bacteriological containers. The product container must be entered aseptically to transfer the product into the bacteriological containers. Challenge microorganisms are added to each container at a concentration of 1 x 105 and 1 x 106 CFU/mL of the product. For antacids (Category 4 products), challenge microbes are added between 1 x 103 and 1x 104 CFU/mL of the product. Containers are then incubated and microbe recovery checked (in CFU via plate-count) for the time points specified in Table 3 below. Microbial plate counts are always completed in duplicate, with the recorded CFU being the average value of the two plates.
Compare the original microbial concentration (in CFU/mL) at the start of the test to recovered microbial concentrations at various time points to determine each microbe’s concentration change as a log reduction value. The log reduction is the difference between the log10 unit value of the starting concentration (CFU/mL) in the suspension and the log10 value (CFU/mL) of the live organisms at the recovery time point.
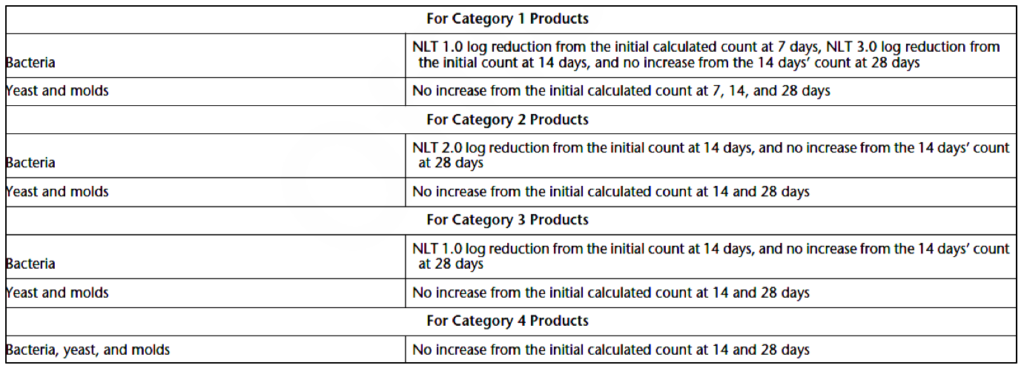
Criteria for Antimicrobial Effectiveness
Antimicrobial agents meet regulatory requirements if the criteria specified in Table 3 are met. A “no increase” in counts is considered not more than 0.5 log10 unit more than the initial microbial concentration to which it is compared. Also, NLT stands for “not less than.”
Summary
Overall, antimicrobial preservatives are agents added to aqueous products to prevent microorganism growth. Antimicrobial testing assesses how effective antimicrobial agents are at preventing the growth of microbial contaminants (e.g., bacteria, mold, and yeast). All injectable products packaged in multidose containers must demonstrate antimicrobial effectiveness, whether the antimicrobial activity comes from inherent characteristics of the product formulation or from the addition of a known antimicrobial agent. Aqueous-based, multidose topical and oral formulations must also have antimicrobial effectiveness. Antimicrobial testing involves challenging a product with microbes, promoting microbial growth, and then recovering the microbial concentration at various time points. Microbes used for the challenge are likely contaminants to the product during production or intended use. Ensure you choose a contract manufacturing organization that can support you with appropriate microbiological testing for your unique parenteral product, pharmaceutical formulation, or cosmetic product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <51> Antimicrobial Effectiveness Testing. Rockville, MD, USA. 2021. (USPC <51>).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Sharing this in your social netwroks

