Preservative Challenge Testing Vs. Disinfectant Challenge Testing
What is preservative challenge testing (preservative efficacy testing)?
Preservative challenge testing, also known as preservative efficacy testing (PET), determines the effectiveness of a preservative during its shelf life and evaluates how well a product withstands microbial contamination during use. Preservatives are substances mixed into creams, gels, and other liquids. Preservatives are used to prevent the growth of bacteria, fungi, and other microorganisms within a medical, cosmetic, household, or food product. Preservatives also help keep the freshness of the appearance of a product and keep its consistency intact over time. Cosmetics use preservative challenge testing (preservative testing cosmetics). Baby or medical wipes use preservative challenge testing, and in some cases, disinfectant validations.
What products require preservative challenge testing?
Preservative challenge testing can be used to determine the best preservative to use in your topical formulation and the minimum effective concentration needed to preserve the pharmaceutical, food, or biotechnology product being assessed. Topical formulations (gels, creams, ointments, and lotions) comprise a water or oil base that delivers various medications, natural substances (such as herbs), or moisture through the skin. Topical cosmetics contain preservatives to support the stability of the formulation and prevent microbial growth during repeated product use. Preservative challenge testing is also used for parenteral products containing multiple doses. In these products, antimicrobial preservatives inhibit the growth of any microorganisms introduced during repeated insertion and withdrawal to load individual amounts.
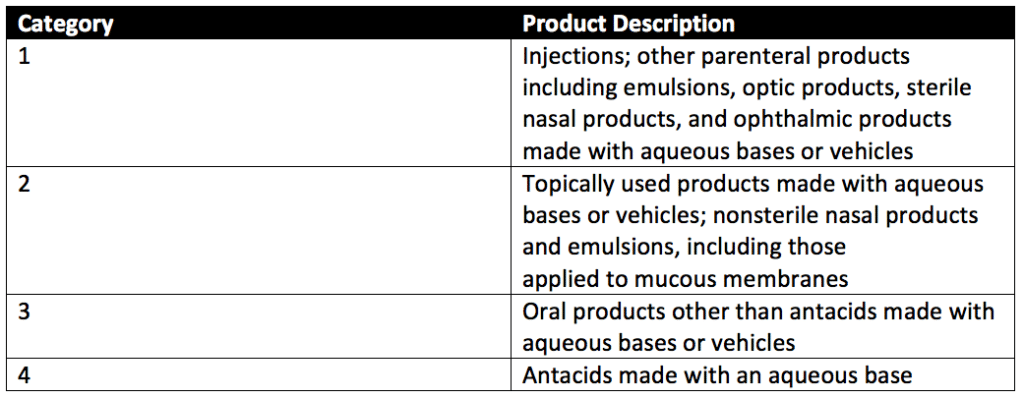
Preservative efficacy testing can evaluate four categories of products with preservative challenge testing. These product categories are detailed in Table 1 of USP 51, reproduced below. Preservative testing cosmetics falls under Category 2 (topicals) most frequently. Baby wipes that use disinfectant validations also fall under Category 2 products.
What is a disinfectant?
A disinfectant is an agent (chemical or physical) that kills or removes harmful microorganisms when applied to a surface. A disinfectant’s effectiveness depends on its natural biocidal activity.
A disinfectant’s ability to kill microbes depends on:
- Concentration
- Contact time with the surface
- Surface type disinfected
- The hardness of the water used for dilution
- Quantity of organic material present on the surface
- Microbial types present
- Number of microorganisms populating the surface
What are disinfectants used for, and why are they important for sterile products?
Validated cleaning and sanitization processes are needed for cleanrooms and other controlled manufacturing environments to prevent microbial contamination of medical and pharmaceutical products. Microbial contamination can occur in various ways during device and drug manufacture. Primary sources of microbial contamination risk include raw ingredient sources, process water, packaging components, processing equipment, and manufacturing operators. Thus, appropriate environmental monitoring that follows current Good Manufacturing Practice (cGMP) is vital to keeping potential contamination risk for health care products and devices low. GMP cleaning and sanitization programs apply to both sterile and nonsterile products.
Disinfectant validations are used for household products and medical products. Disinfectants are also used to keep manufacturing environment surfaces free from harmful contaminants. Disinfectants may be used on floors, walls, and ceilings. Each disinfectant has a certain bacteria-killing (bactericidal), fungi-killing (fungicidal), and sporicidal efficacy that must be verified through a disinfectant validation. Agents that kill bacteria and spores are particularly good at preventing large-scale bacterial growths known as biofilms. Disinfectant selection is based on the manufacturing area’s bacterial, fungi, and spore risk where the disinfectant will be used. In addition to its microbe-killing profile, a disinfectant should keep the drug product or device under manufacture in mind and should not be harmful to the drug chemistry, drug toxicity, or device materials. Disinfectants are inherently toxic, so drug products must be protected from exposure to most disinfectants used. Any changes in the disinfectant initially selected will result in the need for additional disinfectant validations to be performed.
How is preservative challenge testing performed?
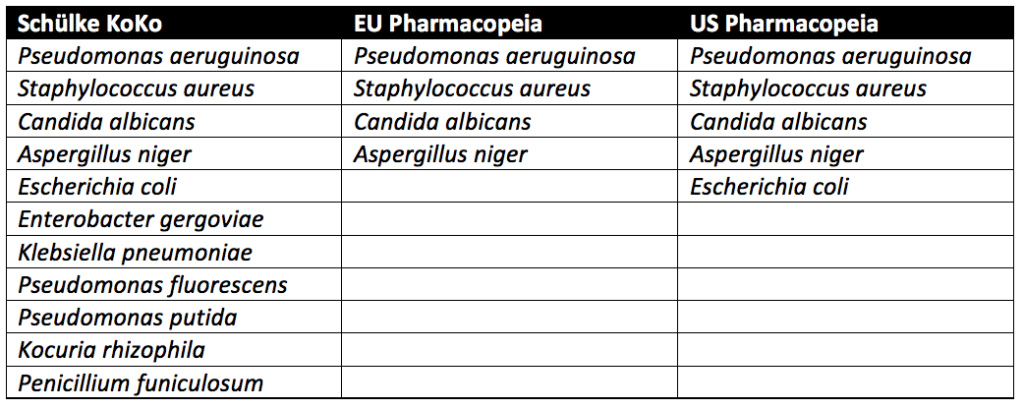
Three approaches are used in preservative challenge testing. These approaches come from the Schülke KoKo Test, United States Pharmacopeia (USP), and the European Pharmacopeia. Any of the three methods described below may be used for preservative testing cosmetics.
Schülke KoKo Test vs. U.S. Pharmacopeia vs. European Pharmacopeia
Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus niger, and Candida albicans must be tested for in all cosmetic products sold in the European Union. In addition to the microbes above, testing with microbes known to lead to spoilage of cosmetic products is recommended but not required. In the United States, the USP guidelines require testing of Escherichia coli in addition to Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus niger, and Candida albicans. Spoilage microbes are not needed for PET. In contrast to the pharmacopeia tests, which only evaluate pathogenic microbes, the Schülke KoKo test evaluates product spoiling microorganisms. Schülke KoKo test’s spoiling microorganisms are based on decades of cosmetic testing experience from Schülke & Mayr in Germany.
Preservative testing cosmetics occurs by exposing them to a single microbial strain at a time. Additional details on USP preservative challenge testing, which follows similar procedures to the European Pharmacopeia, can be found through reading our articles on Preservative Challenge Testing And USP 51 and Preservative Efficacy Testing For Medical Devices. In contrast, for the Schülke KoKo test, the single cultivated microbes are brought together into a mixed suspension to challenge the cosmetic product. A new mixed suspension is prepared for each of the six inoculation cycles for Schülke KoKo testing. Since the Schülke KoKo test uses a mixed microbe inoculation, it simulates microbial exposure of a product during production, filling, and use. Indeed, a product would likely be exposed to multiple microbes at once instead of one at a time. Overall, though different from the USP and European Pharmacopeia methods, the Schülke KoKo Test is a reliable test method for assessing the efficacy of antimicrobial preservation of cosmetic products.
First, cultures of Candida albicans (ATCC No. 10231), Aspergillus brasiliensis (ATCC No. 16404), Escherichia coli (ATCC No. 8739), Pseudomonas aeruginosa (ATCC No. 9027), and Staphylococcus aureus (ATCC No. 6538) are prepared. Stock cultures of these organisms are prepared by centrifuging an ATCC culture removing residual media from the prior culture, and resuspending the microorganisms in a sterile suspension fluid. The suspension fluids for each microorganism referenced above are prepared at a microbial count of about 1 × 108 colony-forming units per milliliter (CFU/mL).
Preservative efficacy testing (preservative testing cosmetics) is performed in five sterile, capped containers. Suppose the product’s original container is sterile, can be entered aseptically, and holds an appropriate product volume. In that case, the original filled product containers may be used. Each of the five product samples is injected with a test suspension of either Candida albicans, Aspergillus brasiliensis, Escherichia coli, Pseudomonas aeruginosa, or Staphylococcus aureus. Note that each product sample is exposed to only a single microbe of the five listed, and all microbes are exposed to the product during testing. The microbial test suspension injected is between 0.5%- 1% of the volume of the product under assessment and is at a concentration of 1 × 105 and 1 × 106 CFU/ml of the product for category 1-3 products. The final concentration per ml of product for category 4 products is between 1 × 103 and 1 × 104 CFU/mL.
Each of the five product samples with their respective microbial injections is incubated at a simulated room temperature of 22.5 ± 2.5°C or 32.5 ± 2.5°C depending upon the microbe the product sample is exposed. Microbial counts are taken at 7, 14, and 28 days for most microorganisms tested. The plate-count method is used to determine the number of CFU present in each of the inoculated product samples. This plate-count method is completed in duplicate. Once all counts are taken, the change in log10 of the concentration of CFU/ml is calculated for each microorganism. The requirements for antimicrobial effectiveness (preservative testing cosmetics) are met if no increase in microbial growth for the various organisms tested occurs within the timeframes specific to each organism. are met. No increase in microbial growth is defined as not more than 0.5 log10 units more than the previous microbial growth value.
What is a disinfectant challenge test, and how is disinfectant challenge testing performed?
EPA validated disinfectants, and their directions, are not sufficient for use in GMP manufacturing for pharmaceutical, biotechnological, and medical device industries. Thus, official disinfectant testing must be performed for disinfectants used in manufacturing environments so that these disinfectants meet aseptic and non-aseptic manufacturing cleaning validation criteria. Disinfectant challenge testing is part of the disinfectant validation process. In the United States, use-dilution tests and surface challenge tests are completed for disinfectant challenges. Use-dilution tests screen disinfectants for their efficacy (at various concentrations and contact times) against a wide range of standard test organisms and environmental isolates. In contrast, surface challenge tests use microorganisms based on typical environmental isolates (for the microbial challenge) and apply disinfectants to surfaces at predetermined concentrations with a specified contact time.
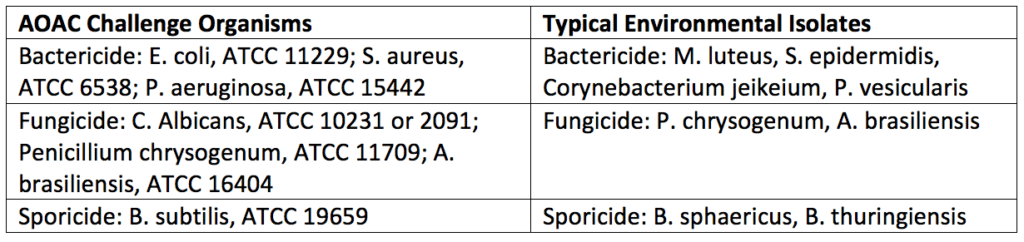
After the surface challenge and use-dilution testing has been performed, a statistical comparison (log reduction) of the numbers of microorganisms isolated before and after disinfection would be completed. Test organisms are counted using swabs, surface rinse, or contact plate methods. As a control, neutralizers that inactivate the disinfectants should be included in either the diluent or microbiological media used for microbe counting. For acceptance criteria, enough organisms need to be killed or removed on a 2-inch × 2-inch square of the surface being decontaminated. Generally, a 2-log reduction (for bacterial spores) or a 3-log reduction (for vegetative bacteria) will need to be accomplished during a predetermined contact time (i.e., 10 minutes). Disinfectants are less effective against the higher numbers of microorganisms used in challenge tests and more effective against the numbers found in clean rooms. Further, the organisms typically used in laboratory tests are more resistant (except for spores) than those used in laboratory tests found in an actual manufacturing environment. Thus, disinfectant challenge tests are robust, and disinfectants that pass these criteria will be more than capable of disinfecting the actual manufacturing area they are selected to maintain. Typical challenge organisms used for disinfectant testing are listed in Table 3 below.
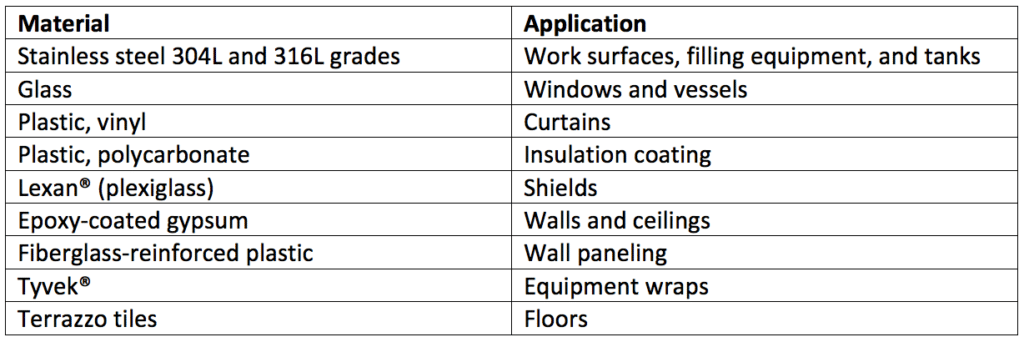
Cleanroom construction isn’t uniform. Thus, each material within the cleanroom and other controlled areas must be evaluated separately to validate the efficacy of a given disinfectant used on its surface. Table 4 is a list of common materials used in cleanroom construction.
What are the differences between preservative and disinfectant challenge testing?
Preservative challenge testing determines the best preservative to use and the minimum effective concentration needed to preserve multidose parenteral products, topicals products, food, or other healthcare items that contain preservatives. In contrast, disinfectant challenge testing uses multiple types of bacteria and fungi to evaluate the effectiveness of a cleaning agent at removing and preventing microbial growth on manufacturing surfaces. Both preservative and disinfectant challenge tests are quantitative and involve testing surfaces or products with various microbes. Further, microbial counting methods for both challenge tests are similar. However, preservative challenge testing evaluates the antimicrobial properties of a product, while disinfectant challenge testing assesses the functionality of various disinfectants used for sterile and non-sterile GMP product manufacturing.
Summary
Overall, preservative challenge and disinfectant challenge testing are imperative for regulatory approval of pharmaceutical/cosmetic preservatives and manufacturing sterility, respectively. These tests ensure that parenteral products, cosmetics, and medical devices have sufficient antimicrobial activity and manufacturing sterility to keep patients safe during product use. Preservative efficacy testing evaluates injectables, topicals, orals, and antacids made with an aqueous base for their antimicrobial activity. In contrast, disinfectant challenge testing keeps manufacturing environment surfaces free from harmful contaminants and is a part of GMP-cleaning validations. All in all, ensure you choose a contract manufacturing organization that can support you with appropriate cleaning validations and preservative challenge testing for your unique cosmetic, pharmaceutical, or medical product needs.
MycoScience is a contract manufacturing organization specializing in Preservative Efficacy Testing and Microbial Aerosol Challenge Testing. MycoScience also offers Sterilization Validations, Bioburden Testing, Cleaning Validations, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <51> Antimicrobial Effectiveness Testing. Rockville, MD, USA. 2021. (USPC <51>).
United States Pharmacopeial Convention. <1072> Disinfectants And Antiseptics. Rockville, MD, USA. 2021. (USP <1072>).
United States Pharmacopeial Convention. <1207.2> Package Integrity Leak Test Technologies. Rockville, MD, USA. 2021. (USPC <1207.2>).
Sharing this in your social netwroks

