Sterilization vs. Sanitization vs. Disinfection
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products that undergo sterilization are often chemical, heat, or filter sterilized. Sterilization kills any microorganisms inside the products obtained during manufacturing. For chemical and heat sterilization, sterilization occurs after the product is placed in its final packaging. For sterilization by filtration, the product is often filtered and then aseptically filled into a sterile container. The last sterilization process after manufacturing is known as terminal sterilization.
There are four main methods of sterilization:
- Heat
- Gas
- Radiation
- Filtration
Another way to classify these four sterilization methods is:
- Thermal
- Moist
- Dry
- Nonthermal
- Filtration
- Radiation
- Chemical
- Gaseous
- Liquid
What is the difference between sterile and aseptic?
While both sterile and aseptic products will prevent microbial contamination following use, the processes by which microbial contamination is prevented are different. An aseptic process prevents contamination by the exclusion of microorganisms. In contrast, products created using a sterile process use a terminal sterilization process to kill live microbes before product use. Though the definitions for aseptic and sterile are not the same, sterile is used interchangeably with aseptic. Indeed, many products labeled as sterile are manufactured by aseptic processing rather than terminal sterilization.
What is sanitization?
Sanitization reduces the microbial population. However, unlike sterilization, sanitization does not aim to eliminate the microbial population by a specific amount. Thus, sanitizing agents are NOT sterilizing agents.
What is disinfection?
A disinfectant is used to kill potentially infectious agents. Disinfectants are also NOT sterilizing agents.
What are sanitization and disinfection agents?
Antimicrobial liquid agents are classified either as a sanitizing solution or a disinfectant solution. The title “sanitizing” or “disinfectant” describes the role of the solution more than the solution’s capacity to reduce a microbial population. The FDA considers a sanitizer as an agent used to reduce microbial contamination on inanimate environmental surfaces. A disinfectant kills microbes that cause infectious diseases (such as bacterial infections or viruses). Sanitizing agents should be chosen based on their effectiveness against microorganisms in the environment they are sanitizing. Table 13-1A below lists a variety of common sanitizing agents. Sanitizing agents are not sporicidal. Thus, sporicidal agents will need to be separately selected and used for harsh environments that could house bacterial spores. A list of
common sporicidal agents used in medical product manufacturing facilities is listed in Table 13-1B below. Note that sanitizing agents support manufacturing environment cleanliness but are not substitutes for good housekeeping, manufacturing environment controls, and environmental monitoring design.
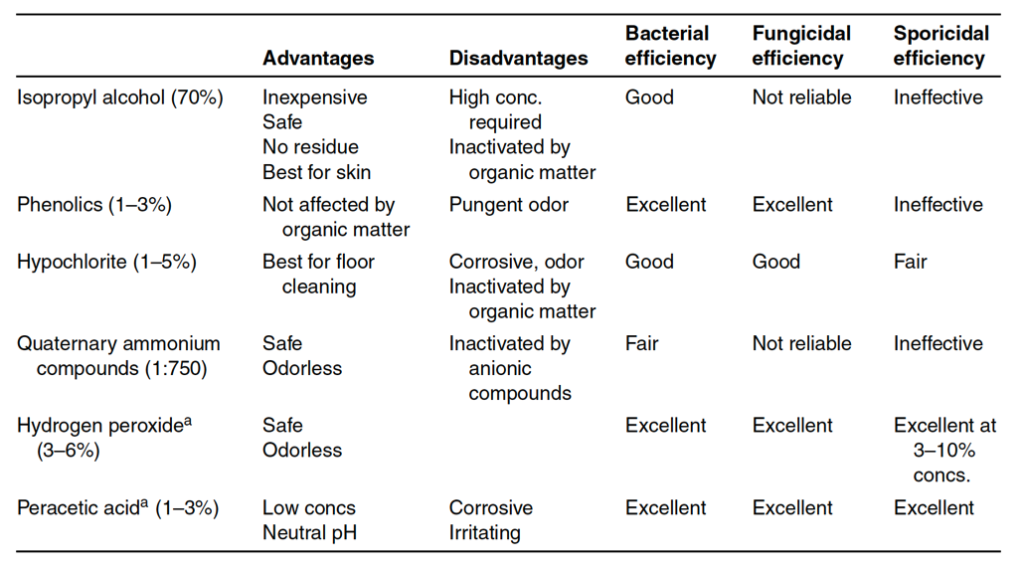
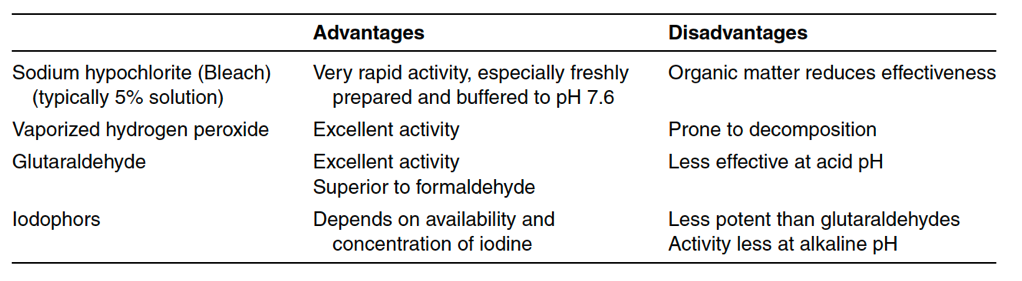
Validation for sanitizing agents involves the collection of data for reproducible bactericidal (or sporicidal) activity. Microorganisms used in validation are microbes isolated from the manufacturing environment. A two or three-log reduction during a microbial challenge is required for a sanitizing agent to be effective. Validation test methods use membrane filters or surface testing. Sanitizing chemical effectiveness depends on sanitizing agent concentration, exposure time, pH, and temperature. The presence of any organic materials is also relevant during testing as many organic materials inactivate sanitizing agents. Further, disinfectants and sanitizing agents should be rotated as needed to prevent microorganism resistance to sanitizing agents over time. Overall sanitizing solution effectiveness comes from the chemical destruction of the microorganisms by the agent and the physical removal of the microorganisms when using the sanitizing agents on hard surfaces. Sanitizing agents are commonly used for floors, walls, equipment surfaces, workstations, chairs, communication systems, and other surfaces that cannot be sterilized. Floors, in particular, may be sanitized multiple times per day. Sanitizations fall into three categories of use: deep-cleaning, routine, and continuous. Deep-cleanings are less frequent and commonly completed after an area shutdown. Routine sanitization ranges between from daily to weekly in frequency, whereas continuous sanitization protocols happen multiple times per day. The frequent sanitization of gloved hands and utensils used during manufacturing are examples of sanitization processes that are continuous.
Ultraviolet (UV) light rays of 237.5 nm wavelength are effective surface disinfectants and alternatives for chemical disinfection. An irradiation intensity of 20 w/cm2 is needed for effective antibacterial activity. However, the drawbacks to using UV as a surface disinfectant include a lack of disinfectant effect in shadow areas and the intensity of the UV reducing by the distance from the source. Other drawbacks are that UV exposure must occur for a sufficient time to kill any offending microbes, particulates in the ray path can reduce UV intensity, and UV can be deleterious to the epithelium of human eyes. For these reasons, chemical disinfectants are often preferred.
Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization for medical devices and products is critical for ensuring patient safety during product use. Sanitization reduces the microbial population. However, unlike sterilization, sanitization does not aim to eliminate the microbial population by a specific amount. Antimicrobial agents are classified either as a sanitizing solution or a disinfectant solution. The title “sanitizing” or “disinfectant” describes the role of the solution more than the solution’s capacity to reduce a microbial population. The FDA considers a sanitizer as an agent used to reduce microbial contamination on inanimate environmental surfaces. A disinfectant is generally used to kill microbes that cause infectious diseases (such as bacterial infections or viruses). Sanitizing and disinfectant agents should be validated and chosen based on their effectiveness against microorganisms that exist in the manufacturing environment they are sanitizing. Whether you are dealing with sterilization validation or cleaning validations, ensure you choose a contract manufacturing organization that can support you with your unique medical device or product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Sharing this in your social netwroks

