How to perform cleaning validations for glassware
What are cleaning validations?
Cleaning validations are validated processes that meet regulatory acceptance criteria for washing, rinsing, disinfection, sanitization, and other cleaning processes for current good laboratory practice (cGMP) manufacturing surfaces, equipment, and environments. Cleaning validations are performed for both aseptic and non-aseptic manufacturing processes.
Why are cleaning validations for glassware important?
When it comes to regulatory testing, successful sample collection and assay performance rely on the glassware’s cleanliness. Examples of tests where clean glassware is critical for an accurate outcome include bacterial endotoxin testing, total organic carbon tests, and heparin, sodium, and vitamin B12 activity assays. Cleaning validations are also critical to prepare glassware for sterilization procedures so that glassware may be used to house sterile products. Cleaning protocols may use commercial detergents or inorganic reagents for cleaning depending on the intended use of the glassware. Validated cleaning protocols include a statement describing how the success of the cleaning procedure is assessed.
How do you assess a cleaning validation?
The validation of a cleaning process requires qualitative and quantitative assessments of cleaning performance. Occasionally, other assessments are needed to ensure the cleanliness of the item, surface, or environment is appropriate for the intended use. Note that a previously validated cleaning process will need revalidation if significant alterations to the procedure are made or (as is the case with glassware) when the intended use of the cleaned item changes.
How do you perform a qualitative assessment of a glass cleaning process?
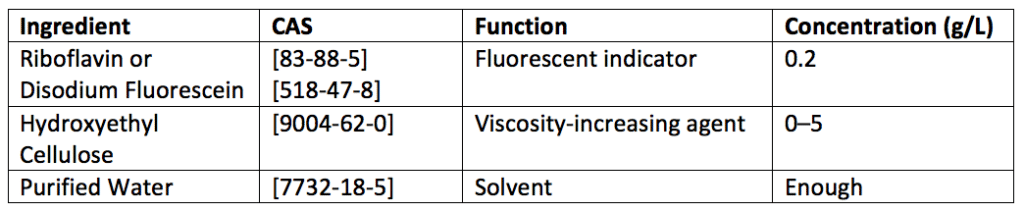
A fluorescent dye or indicator is commonly used for qualitative assessments. The presence or total removal of the fluorescent agent after a cleaning process or procedure is a qualitative measure of the effectiveness of the cleaning process. Riboflavin and fluorescein, two popular fluorescent indicators, are normally used for qualitative assessments. Table 1 describes concentrations needed to prepare fluorescent indicator solutions for fluorescein and riboflavin. Indicator solutions are prepared with little or no viscosity-increasing agent (e.g., hydroxyethylcellulose [HEC]) if the solution will fill the equipment to be cleaned. However, a viscosity-increasing agent is added if the solution will be sprayed into or onto the equipment undergoing cleaning. The concentration of the fluorescent indicator used for qualitative assessment is increased if additional cleanliness sensitivity is required. The presence or absence of the fluorescent indicator is assessed using a black light or ultraviolet (UV) lamp with excitation of about 365 nanometers.

Qualitative cleaning assessment basics:
- Take a dirty, worst-case glassware load or prepare a worst-case load by applying a fluorescent indicator (see Table 1).
- Inspect glassware with a UV lamp or black light to verify worst-case load soil or fluorescent indicator coverage.
- Perform cleaning with the process to be validated.
- Inspect glassware with a UV lamp or black light to qualitatively evaluate cleanliness.
How do you perform a quantitative assessment of a glass cleaning process?
For quantitative cleaning assessment, rinse the glassware with purified water after the cleaning process is complete and collect the rinse water. Next, the rinse water is evaluated for any contaminants. The rinse water properties should not have changed after contact with the glassware and should meet the glassware’s cleanliness acceptance criteria to pass a quantitative assessment. The intended use of the glassware and the risks any contaminants create for that intended use should determine the cleanliness level.
Quantitative cleaning assessment basics:
- Rise glassware with purified water
- Collect rinse water
- Test for total organic carbon and water conductivity
How to perform additional cleaning validation analyses for glassware?
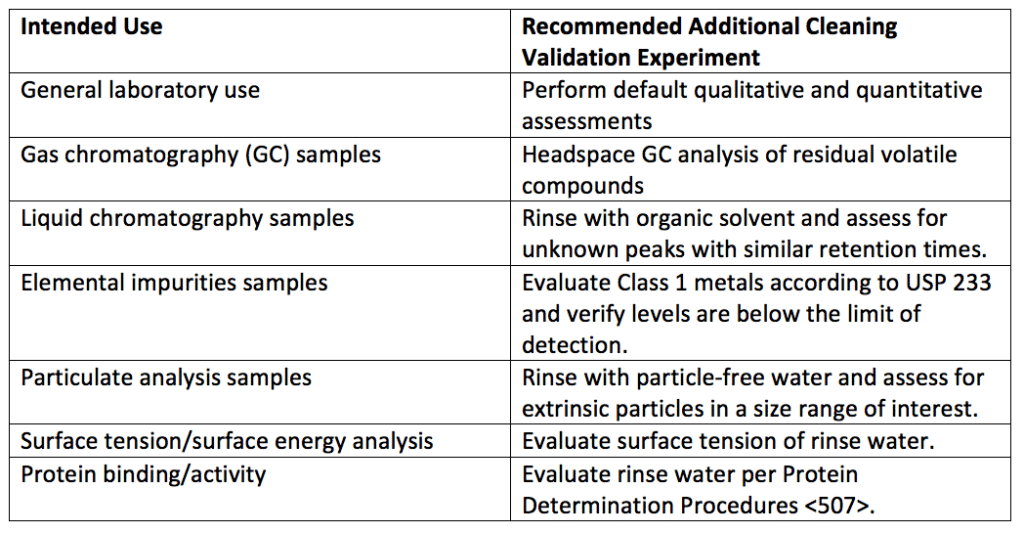
Depending on the intended use of the glassware, cleanliness measurements outside of traditional qualitative and quantitative assessments are used. For example, glassware that will contain gas chromatography (GC) samples should be free of residual volatiles, as any residual volatile agents may interfere with the GC method. A headspace GC analysis can confirm the presence or absence of residual volatiles on the glassware. See Table 2 for additional validation experiments for different glassware uses.
How do you validate automated glassware cleaning?
For automated glassware cleaning, the effectiveness of the cleaning cycles must be validated. First, to validate the cleaning cycle, select a suitable “worst-case scenario” molecule to soil the glassware with. A suitable molecule will represent a worst-case scenario of the materials the glassware may be exposed to (e.g., oil or molecules with poor solubility in common solvents). Next, select several different shaped and sized glassware (e.g., beakers, conical flasks, etc.) and spike the glassware with the worst-case scenario molecule. Then allow the glassware to dry. Next, take the spiked glassware and distribute them in different positions within the automated washer. Fill the washer in with unspiked glassware so that the washer is full. Then run the wash cycle. When the wash cycle is complete, all spiked items are extracted and analyzed quantitatively to determine the residual contaminants on each item. If cleaning acceptance criteria are met after multiple spiked cycles, the cleaning process is considered valid.
Summary
Overall, cleaning validations are performed for both aseptic and non-aseptic manufacturing processes. When it comes to regulatory testing, successful sample collection and assay performance rely on the glassware’s cleanliness. Cleaning validations are also critical to preparing glassware for sterilization procedures. Sterile glassware is often used to fill sterile products. The validation of a cleaning process requires qualitative and quantitative assessments of cleaning performance. Qualitative assessments use the presence of fluorescent dyes to assess cleanliness after a cleaning process. Post-cleaning process rinse water is assessed for contaminants for quantitative glassware assessments. In some glassware applications, such as use for gas chromatography, glassware must undergo additional cleanliness validations to ensure accurate experimental results. All in all, ensure you choose a contract testing organization that can support you with appropriate cleaning validations for your unique medical device or product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1051> Cleaning Glass Apparatus. Rockville, MD, USA. 2021. (USPC <1051>).
Sharing this in your social netwroks

