Top 4 Filters For Parenteral Product Sterilization
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products that undergo sterilization are often chemical, heat, or filter sterilized. Sterilization kills any microorganisms inside the products obtained during manufacturing. For chemical and heat sterilization, sterilization occurs after the product is placed in its final packaging. For sterilization by filtration, the product is often filtered and then aseptically filled into a sterile container. The last sterilization process after manufacturing is known as terminal sterilization. Sterile filters for sterilization including microfilters, particle filters, ultra filters, and nano filters are all covered in this article.

What is sterile filtration?
Sterilization by filtration is a “cold” method of sterilization that removes microbes instead of killing them. Since sterilization by filtration works by removing microbes, sterilization by filtration (also called sterile filtration) is the only sterilization method that doesn’t rely on an elevated temperature, toxic chemicals, or another form of energy (such as gamma radiation) to destroy microorganisms. Sterile filtration is excellent for products that cannot be sterilized with heat or products containing a biological agent, such as an antibody or enzyme.
What are the four primary filter types used for parenteral products and biopharmaceuticals?
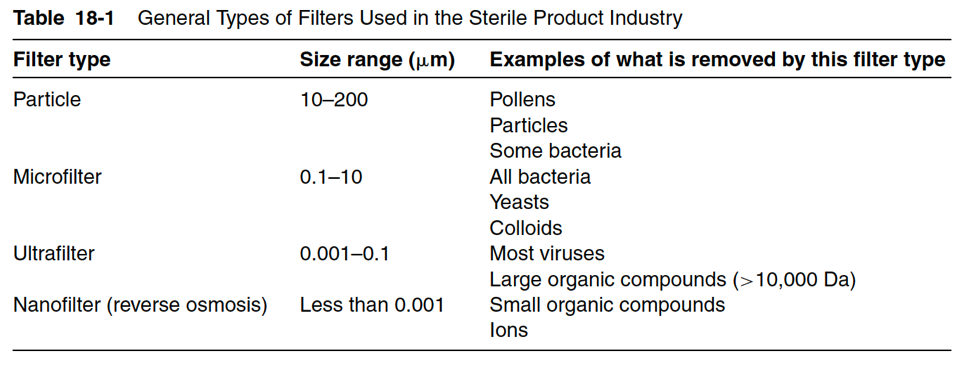
Filters are rated either nominally or absolutely. Nominal ratings are most common and detail the percent removal of particles and bacteria of specific size by weight. For example, a filter with a nominal rating of 67% for 1 micron or greater means that the filter will remove 67% of all particles greater than or equal in diameter to 1 micron. These nominal ratings are based on filter manufacture data, where filters are tested to remove particles from samples with known particle sizes at different weights. The filter ratings for each of the four primary filter types (particle, micro, ultra, and nano) are described in further detail below and applications for these various filters.
#1: Particle Filters
The porosity rating of particle filters ranges from 10 to 200 microns. Particle filters are used as prefilters to remove bulk dirt, pollen, some bacteria, and large particles. Materials used for particle filters (sometimes called depth filters or surface filters) include cellulose, cellulose ester, heat-bonded polypropylene, diatomaceous earth, glass, sand, gravel, and polypropylene yarn. Prefilters are traditionally used to prevent the membranes of microfilters from clogging too quickly.
#2: Microfilters
Most microfilters have a porosity of 0.22 microns or smaller. However, microfilter porosity ranges from 0.1 to 10 microns. Microfilters are used to remove all bacteria and yeast. Microfilters also remove colloidal forms in suspension. Table 18-1 details the typical materials used to create microfilters. As the industry’s classic sterilizing filter, microfilters have narrow pore distribution resulting from the creation of controlled polymeric structures. Some combination filters use microfilter pore sizing along with a particle filter. These combination filters are ubiquitously used as final filters for syringes before product administration.
#3: Ultra filters
Ultra filter porosity ranges from 0.001 to 0.1 microns. Ultra filters are viral filters designed to filter viral particles and large organic compounds (compounds greater than 10,000 Daltons).
#4: Nano-filters
Nano-filter porosity is less than 0.001 microns and is used to remove small organic compounds
and ionic particles. Nano-filters are used in reverse osmosis systems. Nano-sized activated alumina particles bonded to glass fiber matrices, polycarbonate, electrospun Nylon 6 fibers, polyethersulfone, and other polymers are used to create nano-filters. Note that polymeric filter materials are either hydrophilic or hydrophobic. Hydrophilic filters wet spontaneously and are used in sterile filtration of solutions. Hydrophobic filters don’t wet spontaneously. Thus, hydrophobic filters are used to filter gases, solvents, and strongly acidic or alkaline solutions.
Summary
Overall, sterilization by filtration sterilizes parenteral products by removing microbes, particulate matter, and viruses from the manufactured product. There are four primary filters used for sterilization: particulate filters, microfilters, ultrafilters, and nano-filters. Particulate filters have the greatest porosity, while nano-filters have the smallest porosity. Microfilters are used to remove most bacteria and yeast, while ultrafilters remove most viral particles. All in all, product sterility ensures that patients will not be at risk of infection following product use. Ensure you choose a contract testing organization that can provide appropriate sterilization validations for your parenteral product needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling. MycoScience also offers Preservative Efficacy Testing, Sterilization Validations, Bioburden Testing, Cleaning Validations, Microbial Aerosol Challenge Testing, Accelerated Aging, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Sharing this in your social netwroks

