What Are Method Suitability Testing & Method Equivalency Testing?
What is method suitability?
Method suitability determines if the validated method used for regulatory testing was suitable for the new product. For suitability testing, there must be an absence of product effect that would cover up or influence the outcome of the method. Thus, each product tested with certain methods requires appropriate product sample preparation, testing equipment sensitivity for the product, product units, product quantities, and reagent selection/sensitivity.
How is method suitability demonstrated for quantitative and qualitative test methods?
Method suitability is proven using three independent tests: accuracy validation, precision validation, and recovery of challenge organisms. Only quantitative methods need accuracy and precision validation parameters. Proving challenge organism recovery (e.g., USP 62, USP 71, and USP 1227) is enough to show method suitability for qualitative methods.
What is an example of method suitability testing?
With a nucleic acid test method as an example, suitability tests for each new product must be completed to prove that the residual product doesn’t interfere with the target nucleic acid’s concentration, extraction, purification, and recovery. Additionally, the residual product must not interfere with the polymerase chain reaction (PCR) amplification or the chemical probe detection of the target ribosomal ribonucleic acid (rRNA) gene sequence.
What do microbiology tests evaluate, and how does this relate to equivalency testing?
The signal used to estimate a sample’s microbial concentration in an alternative test method is often not colony-forming units (CFUs) like in compendial methods. Thus, it is essential to keep in mind that every microbiological test performed is designed to help companies decide the microbiological quality of a product, raw material, component, or process step. With the overarching goals of microbial testing in mind, alternative methods for equivalency testing may evaluate the presence of organisms, the absence of microorganisms, or estimate the number of organisms present in a sample.
What is method equivalency testing?
Method equivalency testing determines whether an alternative test method is equivalent to a USP general test method (i.e., USP 51, USP 61, USP 62, USP 63, and USP 71). An alternative method need only demonstrate equivalency to a USP test with one product. However, method suitability will need to be determined for each new product evaluated with the equivalent alternative microbiological method.
Alternative microbial method validation should involve:
- Equivalence demonstration
- Analytical method and equipment qualification
How do you demonstrate method equivalency?
Alternate methods must provide proven advantages in accuracy, sensitivity, precision, selectivity, adaptability to automation, or computerized data reduction before they can be used instead of compendial methods. In other words, alternative methods must produce statistically equivalent or better (non-inferior) results than the compendial method.
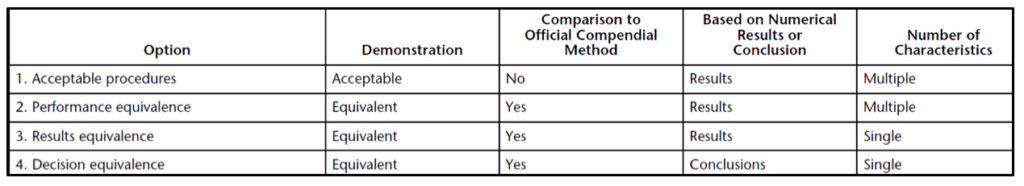
Four options are available to establish alternative analytical method equivalency:
- Acceptable procedures (i.e., meeting a minimum performance or acceptance requirement without a need to demonstrate equivalence to the compendial method)
- Performance equivalence to the compendial method
- Results equivalence to the compendial method
- Decision equivalence to the compendial method
A comparison of the four equivalence options above is shown in Table 1 below.
How to demonstrate equivalence with an acceptable procedure
An acceptable procedure is not strictly an equivalence test comparing an alternative method with a compendial method. In an acceptable procedure, a reference material with known properties may be used to prove the acceptability of the results from an alternative method. Examples of reference materials with known properties are a standard microorganism inoculum and an ATP level. In many cases, the alternative method must measure the signal from the acceptable procedure in the test sample’s presence and with validation criteria consistent with the technology’s scientifically proven capability (e.g., detection limits).
How to demonstrate equivalence with performance equivalence
Performance equivalence requires the alternative method to have equivalent or better results for validation criteria. Examples of validation criteria are test accuracy, precision, specificity, detection limits, qualification limits, robustness, and ruggedness. Sometimes the alternative method may not conform to all validation parameters compared with the USP method. In cases like these, the alternative method is still acceptable if the alternative method’s advantages are robust enough. Improvements in the time to obtain a test result or reductions in the cost to run a test are examples of robust advantages. Alternative methods that can assess the test material quality may also be acceptable, even if they differ from the official method in one or more validation parameters.
How to demonstrate equivalence with results equivalence
The alternative and compendial test methods must provide equivalent numerical results for results equivalence. The same sample cannot be tested in microbiology. Thus, a tolerance interval is established when comparing the alternative and compendial methods. Within the set tolerance interval, the alternative method should be numerically superior or non-inferior. Alternative methods that are non-growth-based may produce significantly higher cell count estimates than CFU growth methods. If the alternative method signal is not in CFUs, a calibration curve showing a correlation between the alternative and compendial test method within the product specification range should be created.
How to demonstrate equivalence with decision equivalence
Decision equivalence is like results equivalence. The difference between decision equivalence and results equivalence is that a pass/fail result (instead of a numerical result) is proven. With decision equivalence, the positive and negative test result frequency should be no worse than (non-inferior to) the frequency of the USP compendial method.
Summary
Overall, method suitability determines if the regulatory testing method was suitable for the new product evaluated. For suitability testing, there must be an absence of product effect that would cover up or influence the outcome of the method. Thus, appropriate product sample preparation, testing equipment sensitivity for the product, product units, product quantities, and reagent selection/sensitivity must occur for the test method for each product assessed. Method suitability is proven using three independent tests: accuracy validation, precision validation, and challenge organism recovery. Only quantitative methods need accuracy and precision validation parameters. Proving challenge organism recovery, as demonstrated in USP 62, USP 71, and USP 1227, is enough to show method suitability for qualitative methods. A method equivalency test determines if a validated alternative microbiological method is equivalent to a USP general test method (e.g., USP 51, USP 61, USP 62, USP 63, and USP 71). Method equivalency can be proven through four means: acceptable procedures, performance equivalence, results equivalence, or decision equivalence to the compendial method. An alternative method need only demonstrate equivalency to a USP test with one product. However, method suitability will need to be determined for each new product evaluated with the equivalent alternative microbiological method. MycoScience can perform FDA-approved suitability testing. Upcoming FDA guidelines will require suitability testing for products (such as general wipes or baby wipes) that have undergone preservative efficacy testing. Ensure you choose a contract testing organization that can support you with appropriate suitability testing for the regulatory needs of your product.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling for parenteral products. MycoScience also offers testing services, including Preservative Efficacy Testing/Suitability Testing, Bioburden Testing, Microbial Aerosol Challenge Testing, Cytotoxicity Testing,Cleaning Validations,Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1223> Validation Of Alternative Microbiological Methods. Rockville, MD, USA. 2022. (USPC <1223>).
Sharing this in your social netwroks

