USP 81 Zone of Inhibition Testing For Antimicrobial Medical Products
What is microbiology testing?
Microbiology testing identifies the presence and the type of microbes in a manufacturing environment, medical product, or medical device. Specific regulatory testing for microbiology may include assays such as microbial enumeration testing, particulate analysis, yeast analysis, antifungal activity assessment, growth promotion testing, microbial limits testing, zone of inhibition testing, water analysis, bacteriostasis/fungistasis testing, bacteria identification, yeast identification, fungi identification, gram-negative staining, product inoculations, biological indicator tests, and BI incubation time reduction studies. Microbiology tests are governed by USP 61 and USP 62. Microbiology testing often requires sample collection from a product, a surface, a water source, or the air. Once samples are obtained, various microbiology testing can be performed. Below are links to articles related to multiple microbiology tests and testing methods. Firstly, further details on USP 788 particulate matter testing can be found here. Microbial characterization methods can be found here, whereas the top techniques for microbiology examination are here. Lastly, information on how to record and evaluate microbiology data is here. This article covers USP 81 zone of inhibition testing (cylinder plate assay) for antimicrobial medical products including zone of inhibition measurements for antibiotic zone of inhibition testing.
What is zone of inhibition testing, and what medical products need zone of inhibition testing?
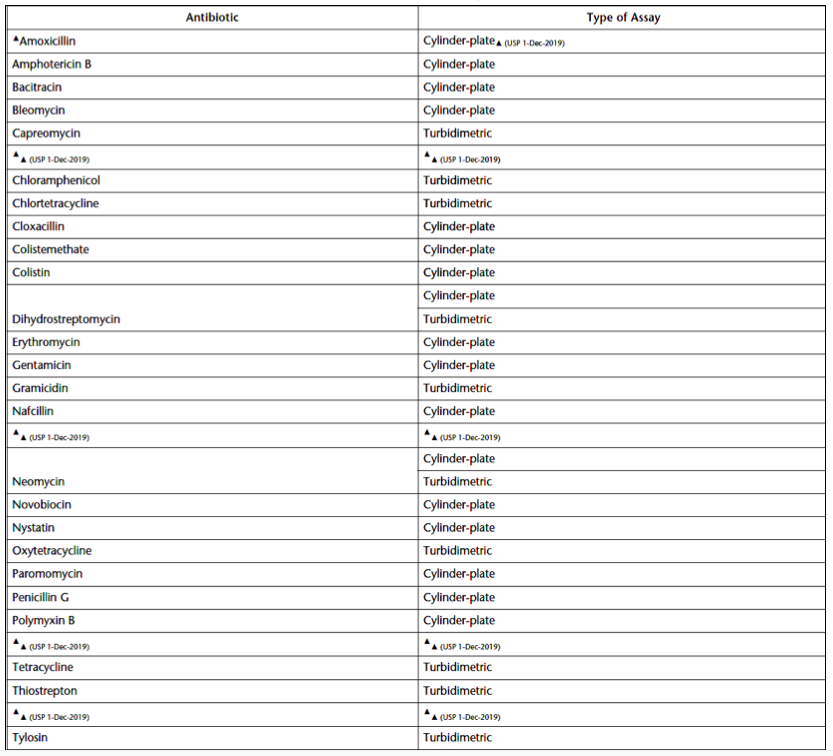
An antimicrobial agent’s potency can be proven by its inhibitory effect on microorganisms. Antimicrobial growth reduction is often assayed using a microbiological zone of inhibition test, as chemical methods can be unreliable. The exception is liquids, gels, and ointments containing preservatives. These antimicrobial medical products should be examined using preservative efficacy testing (PET). USP 81 specifies two methods for zone of inhibition testing: the cylinder-plate (or plate) assay and the turbidimetric (or tube) assay. Table 1 (reproduced from USP 81) lists which antibiotics are assessed with cylinder-plate inhibition testing vs. a turbidimetric assay.
How is a zone of inhibition test performed (USP 81 cylinder-plate method)?
Cylinder-plate testing places a known concentration of an antimicrobial agent (antifungal or antibacterial) or an antimicrobial material of a certain size onto the surface of an agar gel plate containing microbes of known concentration and type. For antimicrobial agents not attached to a material, vertical cylinders are used to keep the microbial concentrate within a specific area. As the antimicrobial agent diffuses into the agar, a measurable “zone” around the cylinder will be created, indicating microbial growth was prevented.
The basic materials required for cylinder-plate testing are disposable Petri dishes with lids and 8-10 millimeters (mm) high porcelain cylinders. A stock solution with a USP Reference Standard of a given antibiotic is prepared in the concentrations specified in Table 2 (reproduced from USP 81). On the day of the zone of inhibition testing, assay five or more test dilutions will be prepared from the stock solution until the final diluent (detailed in Table 2) is reached. The dilution ratio is often a 1:1.25 ratio.
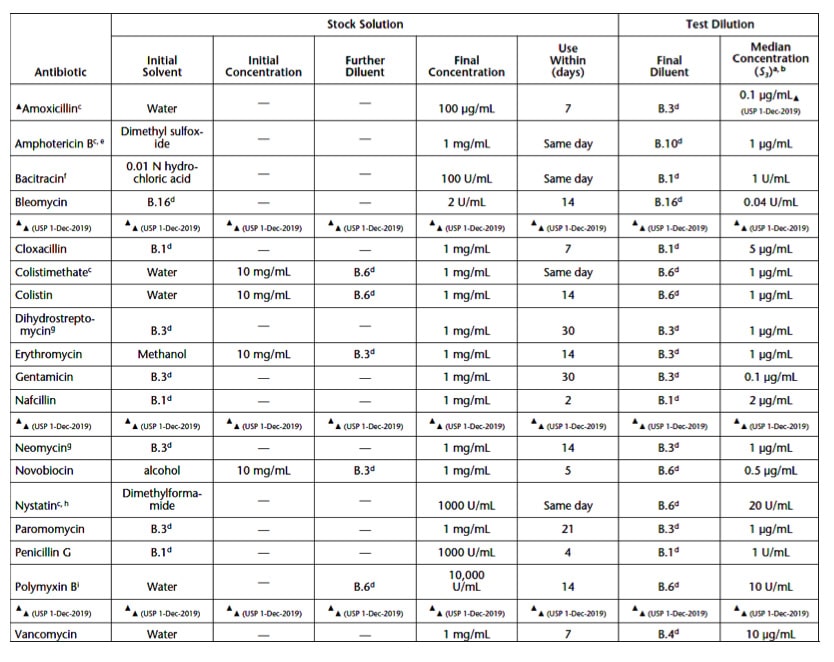
Sample stock solutions (for an antimicrobial agent or antimicrobial coating being tested) are also prepared, mimicking the stock concentrations in Table 2. Potency per unit weight or volume may be used for sample stock solutions. Create a sample stock dilution (U3) from the stock that is the median value of the five USP reference standard stock solution dilutions.
Next, test microorganisms are prepared from a freshly grown slant or culture. The most common organisms for the zone of inhibition assessment are Staphylococcus aureus and Staphylococcus epidermidis. The cultured microorganisms are suspended in sterile saline and plated onto the entire surface of at least two agar plates. Plates are then incubated until growth is apparent. Test microorganisms are then harvested with sterile saline and used to create a stock suspension. A spectrophotometer reading of 25% transmittance at 580 nm is commonly used to create a stock concentration. The goal is to create a stock suspension that results in zones of inhibition of approximately 14–16 mm in diameter for the median concentration of the antimicrobial standard. Zone sizes outside 14-16 mm create assay variability and should be avoided.
For the assay, prepared stock microbes are evenly distributed over cooled agar Petri dishes. Six porcelain cylinders are then added to the inoculated surface of the agar. The cylinders should be evenly spaced across the agar surface. Next, the six cylinders are plated with the same volume of the five USP reference stock dilutions and U3 sample solution. The agar plate is then incubated. After incubation, each zone of inhibition diameter from the cylinders is measured to the nearest 0.1 mm and recorded.
How are cylinder-plate test results assessed?
At least three agar plates with USP reference stock dilutions and U3 sample solution are assessed, and zone of inhibition measurements are made. Zone of inhibition measurements are surface area values quantifying the surface area an anti-microbial agent can inhibit bacteria within. The zone of inhibition measurements can be used to create a standard curve and estimate the potency of the antimicrobial sample compared to the USP reference standard. A standard curve is created by plotting the corrected zone measurements versus the log of the standard concentration values. Calculate the standard curve line equation by performing a standard unweighted linear regression (either with nature log or with base 10 log). The minimum for an accurate percentage coefficient of determination is not less than 95%. To calculate the potency of the product sample, take the antilog and multiply the result by any applicable dilution factor. Potency can also be expressed as a percentage of the reference standard concentration value.

Summary
Overall, zone of inhibition testing is a type of microbiology test that assesses the potency of antimicrobial agents. These antimicrobial agents may be assessed alone, combined within a material, or as a product coating. The use of cylinder-plate assays for zone of inhibition testing is described herein. Cylinder-plate testing places a known concentration of an antimicrobial agent (antifungal or antibacterial) or an antimicrobial material of a certain size onto the surface of an agar gel plate containing microbes of known concentration and type. For antimicrobial agents not attached to a material, vertical cylinders are used to keep the microbial concentrate within a specific area. As the antimicrobial agent diffuses into the agar, a measurable “zone” around the cylinder will be created, indicating microbial growth was prevented. Preservative efficacy testing (PET) may be needed instead of the zone of inhibition testing to verify the potency of antimicrobial preservatives contained within a liquid, gel, or ointment-based cosmetics or medical products. All in all, ensure you choose a contract testing organization that can support you with appropriate microbiology, preservative efficacy, or zone of inhibition testing for your unique cosmetic or medical device needs.
MycoScience is a contract manufacturing organization specializing in sterile syringe and vial filling for parenteral products. MycoScience also offers testing services, including Preservative Efficacy Testing/Suitability Testing, Bioburden Testing, Microbial Aerosol Challenge Testing, Cytotoxicity Testing,Cleaning Validations,Accelerated Aging, Microbiology Testing, EO Residual Testing, Bacterial Endotoxin Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services medical devices and allied industries. MycoScience is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <51> Antimicrobial Effectiveness Testing. Rockville, MD, USA. 2021. (USPC <51>).
United States Pharmacopeial Convention. <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD, USA. 2021. (USPC <61>).
United States Pharmacopeial Convention. <62> Microbiological Examination Of Nonsterile Products: Tests For Specified Microorganisms. Rockville, MD, USA. 2021. (USP <62>).
United States Pharmacopeial Convention. <81> Antibiotics- Microbial Assays. Rockville, MD, USA. 2021. (USP<81>).
Sharing this in your social netwroks

